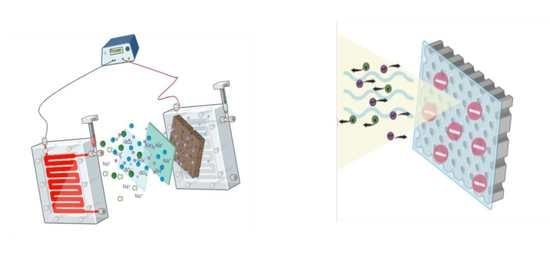
Effects of the Applied Potential on the Performance of Polysulfone Membranes Functionalized with Sulfonated Polyether Ether Ketone Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental
2.2.1. Sulfonation of SPEEK
2.2.2. Membrane Polymer Composite Evolution: 0% to 5% to 10% SPEEK
2.3. Characterization
2.3.1. Cloud Point Measurements
2.3.2. Structural Polymer Evolution
Fourier Transform Infrared Spectroscopy (FT-IR)
X-ray Photoelectron Spectroscopy (XPS) and Depth Profile
NMR Analysis
2.3.3. Morphological Polymer Evolution
Scanning Electron Microscopy (SEM)
Pore Size
2.3.4. Membrane Functional Properties
Zeta Potential
Membrane Wettability
Percent Water Uptake
Ion Exchange Capacity
2.4. Performance Analysis
2.4.1. Constant Pressure Filtration
2.4.2. Constant Flow Filtration
3. Results
3.1. Cloud Point Analysis
3.2. Membrane Characterization
3.2.1. Structural Polymer Evolution
Fourier Transform Infrared Spectroscopy (FT-IR)
X-ray Photoelectron Spectroscopy (XPS) and Depth Profile
NMR Analysis
3.2.2. Morphological Polymer Evolution
Scanning Electron Microscopy (SEM)
Pore Size
3.2.3. Membrane Functional Properties
Zeta Potential/Surface Charge
Wettability
Water Uptake and Ion Exchange Capacity (IEC)
Ion Exchange Capacity (IEC)
3.3. Filtration Studies
3.3.1. Effect of Solvent Evaporation Time on the Performance of the Membrane
3.3.2. Effect of Charge Repulsion
3.3.3. Effects of Applied Voltage Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bamiduro, G.J.; Dollar, C.M.; Abaddi, S.; Ensinger, N.; Zahran, E.M. Rapid photocatalytic mineralization of glyphosate by Pd@BiVO4/BiOBr nanosheets: Mechanistic studies and degradation pathways. Catal. Commun. 2023, 174, 106599. [Google Scholar] [CrossRef]
- van Vliet, M.T.H.; Jones, E.R.; Flörke, M.; Franssen, W.H.P.; Hanasaki, N.; Wada, Y.; Yearsley, J.R. Global water scarcity including surface water quality and expansions of clean water technologies. Environ. Res. Lett. 2021, 16, 024020. [Google Scholar] [CrossRef]
- Anku, W.W.; Oppong, S.O.; Govender, P.P. Bismuth-Based Nanoparticles as Photocatalytic Materials; IntechOpen: London, UK, 2018. [Google Scholar]
- Rajput, R.S.; Pandey, S.; Bhadauria, S. Status of water pollution in relation to industrialization in Rajasthan. Rev. Environ. Health 2017, 32, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Luo, X.; Huang, Y.; Kuang, A.; Yuan, H.; Chen, H. BiOX/BiOY (X, Y = F, Cl, Br, I) superlattices for visible light photocatalysis applications. RSC Adv. 2016, 6, 91508–91516. [Google Scholar] [CrossRef]
- Said, B.; M’rabet, S.; Hsissou, R.; Harfi, A.E. Synthesis of new low-cost organic ultrafiltration membrane made from Polysulfone/Polyetherimide blends and its application for soluble azoic dyes removal. J. Mater. Res. Technol. 2020, 9, 4763–4772. [Google Scholar] [CrossRef]
- Kummu, M.; Guillaume, J.H.A.; de Moel, H.; Eisner, S.; Flörke, M.; Porkka, M.; Siebert, S.; Veldkamp, T.I.E.; Ward, P.J. The world’s road to water scarcity: Shortage and stress in the 20th century and pathways towards sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Chen, Y.; Huang, T.; He, Z.; Xu, J.; Liu, P. Pore Structure and Properties of PEEK Hollow Fiber Membranes: Influence of the Phase Structure Evolution of PEEK/PEI Composite. Polymers 2019, 11, 1398. [Google Scholar] [CrossRef] [Green Version]
- Awad, E.S.; Sabirova, T.M.; Tretyakova, N.A.; Alsalhy, Q.F.; Figoli, A.; Salih, I.K. A Mini-Review of Enhancing Ultrafiltration Membranes (UF) for Wastewater Treatment: Performance and Stability. ChemEngineering 2021, 5, 34. [Google Scholar] [CrossRef]
- Pal, P. (Ed.) Chapter 1—Introduction to the Arsenic Contamination Problem. In Groundwater Arsenic Remediation; Butterworth-Heinemann: Oxford, UK, 2015; pp. 1–23. [Google Scholar] [CrossRef]
- Singh, R. (Ed.) Chapter 1—Introduction to Membrane Technology. In Membrane Technology and Engineering for Water Purification, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2015; pp. 1–80. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kilduff, J.E.; Belfort, G. Modes of Natural Organic Matter Fouling during Ultrafiltration. Environ. Sci. Technol. 2003, 37, 1676–1683. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef]
- Pal, D. Membrane Techniques|Principles of Ultrafiltration. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 3837–3842. [Google Scholar] [CrossRef]
- Nguyen, H.T.V.; Ngo, T.H.A.; Do, K.D.; Nguyen, M.N.; Dang, N.T.T.; Nguyen, T.T.H.; Vien, V.; Vu, T.A. Preparation and Characterization of a Hydrophilic Polysulfone Membrane Using Graphene Oxide. J. Chem. 2019, 2019, 3164373. [Google Scholar] [CrossRef] [Green Version]
- Fane, A.G.; Xi, W.; Rong, W. Chapter 7—Membrane filtration processes and fouling. In Interface Science and Technology; Newcombe, G., Dixon, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 10, pp. 109–132. [Google Scholar]
- Matin, A.; Rahman, F.; Shafi, H.Z.; Zubair, S.M. Scaling of reverse osmosis membranes used in water desalination: Phenomena, impact, and control; future directions. Desalination 2019, 455, 135–157. [Google Scholar] [CrossRef]
- Kwong, M.; Abdelrasoul, A.; Doan, H. Controlling polysulfone (PSF) fiber diameter and membrane morphology for an enhanced ultrafiltration performance using heat treatment. Results Mater. 2019, 2, 100021. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.-B.; Yu, L.-Y.; Wei, Y.-M.; Xu, Z.-L. Structure and Properties of PSf Hollow Fiber Membranes with Different Molecular Weight Hyperbranched Polyester Using Pentaerythritol as Core. Polymers 2020, 12, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Luo, X.-B.; Ding, L.; Luo, S.-L. 4—Application of Nanotechnology in the Removal of Heavy Metal from Water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Luo, X., Deng, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. [Google Scholar] [CrossRef]
- Maria Mahimai, B.; Gandhimathi, S.; Sekar, K.; Kannaiyan, D.; Deivanayagam, P. Sulfonated poly (ether ether ketone): Unprecedented ion-exchange polymer electrolytes for fuel cell applications—A versatile review. Mater. Adv. 2022, 3, 6085–6095. [Google Scholar] [CrossRef]
- Tomasino, E.; Mukherjee, B.; Ataollahi, N.; Scardi, P. Water Uptake in an Anion Exchange Membrane Based on Polyamine: A First-Principles Study. J. Phys. Chem. B 2022, 126, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Ling, X.; Yuan, D.; Cheng, Y.; Wu, C.; Chao, Z.S.; Sun, L.; Yan, C.; Jia, C. SPEEK Membrane of Ultrahigh Stability Enhanced by Functionalized Carbon Nanotubes for Vanadium Redox Flow Battery. Front. Chem. 2018, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Sastri, V.R. (Ed.) Chapter 8—High-Temperature Engineering Thermoplastics: Polysulfones, Polyimides, Polysulfides, Polyketones, Liquid Crystalline Polymers, and Fluoropolymers. In Plastics in Medical Devices; William Andrew Publishing: Boston, MA, USA, 2010; pp. 175–215. [Google Scholar] [CrossRef]
- Fink, J.K. (Ed.) 3—Epoxy Resins. In Reactive Polymers: Fundamentals and Applications, 3rd ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 139–223. [Google Scholar] [CrossRef]
- Zydney, A. Charged Ultrafiltration Membrane. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–2. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Winter, L.R.; Zhao, Y.; Ma, W.; Hedtke, T.; Kim, J.-H.; Elimelech, M. Electrified Membranes for Water Treatment Applications. ACS EST Eng. 2021, 1, 725–752. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. Tailoring Membrane Surface Charges: A Novel Study on Electrostatic Interactions during Membrane Fouling. Polymers 2015, 7, 2017–2030. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Bruening, M.L. Ion separations with membranes. J. Polym. Sci. 2020, 58, 2831–2856. [Google Scholar] [CrossRef]
- Gholami, F.; Asadi, A.; Zinatizadeh, A.A. Efficient heavy metals and salts rejection using a novel modified polysulfone nanofiltration membrane. Appl. Water Sci. 2022, 12, 146. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Y. Covalently crosslinked graphene oxide membranes by esterification reactions for ions separation. J. Mater. Chem. A 2015, 3, 4405–4412. [Google Scholar] [CrossRef]
- Fionah, A.; Hackett, C.; Aljewari, H.; Brady, L.; Alqhtani, F.; Escobar, I.C.; Thompson, A.K. Microcystin-LR Removal from Water via Enzymatic Linearization and Ultrafiltration. Toxins 2022, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Fathima, N.; Rathinam, A.; Lawrence, D.; Yugandhar, U.; Moorthy, T.; Nair, B. SPEEK polymeric membranes for fuel cell application and their characterization: A review. J. Sci. Ind. Res. 2007, 66, 209–219. [Google Scholar]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Ismail, A.F.; Matsuura, T. (Eds.) Sustainable Membrane Technology for Energy, Water, and Environment [Electronic Resource]; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Cadore, Í.R.; Ambrosi, A.; Cardozo, N.S.M.; Tessaro, I.C. Phase separation behavior of poly(ethylene terephthalate)/(trifluoroacetic acid/dichloromethane)/water system for wet phase inversion membrane preparation. J. Appl. Polym. Sci. 2019, 136, 47263. [Google Scholar] [CrossRef]
- Yao, D.; Wei, T.; Shang, L.; Na, H.; Zhao, C. A comparative study of side-chain-type poly(ether ether ketone) anion exchange membrane functionalized with different hetero-cycloaliphatic quaternary ammonium groups. RSC Adv. 2019, 9, 7975–7983. [Google Scholar] [CrossRef]
- Ferlita, R.R.; Phipps, D.; Safarik, J.; Yeh, D.H. Cryo-snap: A simple modified freeze-fracture method for SEM imaging of membrane cross-sections. Environ. Prog. 2008, 27, 204–209. [Google Scholar] [CrossRef]
- Ogbuoji, E.A.; Stephens, L.; Haycraft, A.; Wooldridge, E.; Escobar, I.C. Non-Solvent Induced Phase Separation (NIPS) for Fabricating High Filtration Efficiency (FE) Polymeric Membranes for Face Mask and Air Filtration Applications. Membranes 2022, 12, 637. [Google Scholar] [CrossRef]
- Mosqueda-Jimenez, D.; Narbaitz, R.; Matsuura, T. Membrane Fouling Test: Apparatus Evaluation. J. Environ. Eng. 2004, 130, 90–99. [Google Scholar] [CrossRef]
- Dong, X.; Al-Jumaily, A.; Escobar, I.C. Investigation of the Use of a Bio-Derived Solvent for Non-Solvent-Induced Phase Separation (NIPS) Fabrication of Polysulfone Membranes. Membranes 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combe, C.; Molis, E.; Lucas, P.; Riley, R.; Clark, M. The effect of CA membrane properties on adsorptive fouling by humic acid. J. Membr. Sci. 1999, 154, 73–87. [Google Scholar] [CrossRef]
- Fievet, P.; Szymczyk, A.; Labbez, C.; Aoubiza, B.; Simon, C.; Foissy, A.; Pagetti, J. Determining the Zeta Potential of Porous Membranes Using Electrolyte Conductivity inside Pores. J. Colloid. Interface Sci. 2001, 235, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Saito, T.; Hickner, M.A. Zeta potential of ion-conductive membranes by streaming current measurements. Langmuir 2011, 27, 4721–4727. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Loh, K.S.; Walvekar, R.; Khalid, M. Comparative Study On Water Uptake And Ionic Transport Properties Of Pre- And Post Sulfonated Chitosan/PVA polymer Exchange Membrane. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012017. [Google Scholar] [CrossRef]
- Khan, M.; Mondal, A.; Tong, B.; Jiang, C.; Kamana, E.; Yang, Z.; Wu, L.; Xu, T. Development of BPPO-based anion exchange membranes for electrodialysis desalination applications. Desalination 2015, 391, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Pirali-Hamedani, M.; Mehdipour-Ataei, S. Effect of sulfonation degree on molecular weight, thermal stability, and proton conductivity of poly(arylene ether sulfone)s membrane. Des. Monomers Polym. 2017, 20, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Gruskevica, K.; Mezule, L. Cleaning Methods for Ceramic Ultrafiltration Membranes Affected by Organic Fouling. Membranes 2021, 11, 131. [Google Scholar] [CrossRef]
- Deqian, R. Cleaning and Regeneration of Membranes. Desalination 1987, 62, 363–371. [Google Scholar] [CrossRef]
- El-Gendi, A.; Abdallah, H.; Ali, S. Construction of Ternary Phase Diagram and Membrane Morphology Evaluation for Polyamide/Formic acid/Water System. Aust. J. Basic Appl. Sci. 2012, 6, 62–68. [Google Scholar]
- Tshindane, P.; Mamba, B.B.; Motsa, M.M.; Nkambule, T.T.I. Delayed Solvent–Nonsolvent Demixing Preparation and Performance of a Highly Permeable Polyethersulfone Ultrafiltration Membrane. Membranes 2023, 13, 39. [Google Scholar]
- Ladewig, B.; Al-Shaeli, M. Fundamentals of Membrane Processes. In Fundamentals of Membrane Bioreactors: Materials, Systems and Membrane Fouling; Springer: Berlin/Heidelberg, Germany, 2017; pp. 13–37. [Google Scholar] [CrossRef]
- Mondal, S.; Kumar Majumder, S. Fabrication of the polysulfone-based composite ultrafiltration membranes for the adsorptive removal of heavy metal ions from their contaminated aqueous solutions. Chem. Eng. J. 2020, 401, 126036. [Google Scholar] [CrossRef]
- Guhan, S.; Rethinasabapathy, M.; Dharmalingam, S. Development of a solid polymer electrolyte membrane based on sulfonated poly(ether ether)ketone and polysulfone for fuel cell applications. Can. J. Chem. 2011, 90, 205–213. [Google Scholar] [CrossRef]
- He, S.; Lin, Y.; Ma, H.; Jia, H.; Liu, X.; Lin, J. Preparation of sulfonated poly(ether ether ketone) (SPEEK) membrane using ethanol/water mixed solvent. Mater. Lett. 2016, 169, 69–72. [Google Scholar] [CrossRef]
- Eke, J.; Elder, K.; Escobar, I.C. Self-Cleaning Nanocomposite Membranes with Phosphorene-Based Pore Fillers for Water Treatment. Membranes 2018, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Sari Erkan, H.; Bakaraki Turan, N.; Önkal Engin, G. Chapter Five—Membrane Bioreactors for Wastewater Treatment. In Comprehensive Analytical Chemistry; Chormey, D.S., Bakırdere, S., Turan, N.B., Engin, G.Ö., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 81, pp. 151–200. [Google Scholar]
- Singh, R. (Ed.) Chapter 1—Introduction to membrane technology. In Hybrid Membrane Systems for Water Purification; Elsevier Science: Amsterdam, The Netherlands, 2005; pp. 1–56. [Google Scholar] [CrossRef]
- Kadel, S.; Pellerin, G.; Thibodeau, J.; Perreault, V.; Lainé, C.; Bazinet, L. How Molecular Weight Cut-Offs and Physicochemical Properties of Polyether Sulfone Membranes Affect Peptide Migration and Selectivity during Electrodialysis with Filtration Membranes. Membranes 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiasi, S.; Behboudi, A.; Mohammadi, T.; Khanlari, S. Effect of surface charge and roughness on ultrafiltration membranes performance and polyelectrolyte nanofiltration layer assembly. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123753. [Google Scholar] [CrossRef]
- Selva, T.M.G.; Selva, J.S.G.; Prata, R.B. Sensing Materials: Diamond-Based Materials. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Jose, A.J.; Alagar, M. 3—Preparation and characterization of polysulfone-based nanocomposites. In Manufacturing of Nanocomposites with Engineering Plastics; Mittal, V., Ed.; Woodhead Publishing: Soston, UK, 2015; pp. 31–59. [Google Scholar] [CrossRef]
- Kumar, P.; Bharti, R.P.; Kumar, V.; Kundu, P.P. Chapter 4—Polymer Electrolyte Membranes for Microbial Fuel Cells: Part A. Nafion-Based Membranes. In Progress and Recent Trends in Microbial Fuel Cells; Kundu, P.P., Dutta, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 47–72. [Google Scholar] [CrossRef]
- MacKinnon, S.M.; Fuller, T.J.; Coms, F.D.; Schoeneweiss, M.R.; Gittleman, C.S.; Lai, Y.H.; Jiang, R.; Brenner, A.M. Fuel Cells—Proton-Exchange Membrane Fuel Cells|Membranes: Design and Characterization. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 741–754. [Google Scholar] [CrossRef]
- Grekhov, A.M.; Eremin, Y.S.; Dibrov, G.A.; Volkov, V.V. Percolation of composite poly(vinyltrimethylsilane) membranes with carbon nanotubes. Pet. Chem. 2013, 53, 549–553. [Google Scholar] [CrossRef]
- Kusworo, T.; Yono, B.; Ikhsan, D.; Rokhati, N.; Prasetyaningrum, A.; Mutiara, F.R.; Sofiana, N.R. Effect of combination dope composition and evaporation time on the separation performance of cellulose acetate membrane for demak brackish water treatment. MATEC Web Conf. 2017, 101, 01004. [Google Scholar] [CrossRef]
- Lu, D.; Babaniamansour, P.; Williams, A.; Opfar, K.; Nurick, P.; Escobar, I.C. Fabrication and evaporation time investigation of water treatment membranes using green solvents and recycled polyethylene terephthalate. J. Appl. Polym. Sci. 2022, 139, e52823. [Google Scholar] [CrossRef]
- Oncsik, T.; Trefalt, G.; Borkovec, M.; Szilagyi, I. Specific Ion Effects on Particle Aggregation Induced by Monovalent Salts within the Hofmeister Series. Langmuir 2015, 31, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, H.-L.; Zhang, J.-S.; Lin, C.; Tan, Z.-J. Effective Repulsion Between Oppositely Charged Particles in Symmetrical Multivalent Salt Solutions: Effect of Salt Valence. Front. Phys. 2021, 9, 696104. [Google Scholar] [CrossRef]
- Li, Y.; Girard, M.; Shen, M.; Millan, J.A.; Olvera de la Cruz, M. Strong attractions and repulsions mediated by monovalent salts. Proc. Natl. Acad. Sci. USA 2017, 114, 11838–11843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, H.T.; Bartelt-Hunt, S.; Rodenhausen, K.B.; Schubert, M.; Bartz, J.C. Investigation of Bovine Serum Albumin (BSA) Attachment onto Self-Assembled Monolayers (SAMs) Using Combinatorial Quartz Crystal Microbalance with Dissipation (QCM-D) and Spectroscopic Ellipsometry (SE). PLoS ONE 2015, 10, e0141282. [Google Scholar] [CrossRef]
- Doumas, B.T. Standards for total serum protein assays—A collaborative study. Clin. Chem. 1975, 21, 1159–1166. [Google Scholar] [CrossRef]
- Khan, B.; Haider, S.; Khurram, R.; Wang, Z.; Wang, X. Preparation of an Ultrafiltration (UF) Membrane with Narrow and Uniform Pore Size Distribution via Etching of SiO2 Nano-Particles in a Membrane Matrix. Membranes 2020, 10, 150. [Google Scholar] [CrossRef]
- Bose, J.; Marchio, L.; Adhikari, U.; Datta, D.; Sikder, J. Synthesis and characterization of polyvinylidene fluoride/functionalized silicon carbide nanocomposite membrane for water treatment. J. Polym. Res. 2023, 30, 246. [Google Scholar] [CrossRef]
- Iojoiu, C.; Danyliv, O.; Alloin, F. 16—Ionic Liquids and Polymers for Battery and Fuel Cells. In Modern Synthesis Processes and Reactivity of Fluorinated Compounds; Groult, H., Leroux, F.R., Tressaud, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 465–497. [Google Scholar] [CrossRef]
- Mir, F.; Shukla, A. Negative Rejection of NaCl in Ultrafiltration of Aqueous Solution of NaCl and KCl Using Sodalite Octahydrate Zeolite−Clay Charged Ultrafiltration Membrane. Ind. Eng. Chem. Res. 2010, 49, 6539–6546. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.; Lim, J.H.; Lee, S.J. Development of a Desalination Membrane Bioinspired by Mangrove Roots for Spontaneous Filtration of Sodium Ions. ACS Nano 2016, 10, 11428–11433. [Google Scholar] [CrossRef]
- Gross, D. Electromobile surface charge alters membrane potential changes induced by applied electric fields. Biophys. J. 1988, 54, 879–884. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Seo, E.; Chang, S.K.; Park, T.J.; Lee, S.J. Novel water filtration of saline water in the outermost layer of mangrove roots. Sci. Rep. 2016, 6, 20426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Li, N.; Wang, Q.; Bolto, B. 6—Desalination by pervaporation. In Emerging Technologies for Sustainable Desalination Handbook; Gude, V.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 205–226. [Google Scholar] [CrossRef]















| Membrane Composition | Solvent (NMP) % | Total Polymer % (PSf + SPEEK) | % SPEEK within Polymer |
|---|---|---|---|
| PSf/NMP | 83 | 17 | 0 |
| 5% SPEEK-PSf/NMP | 83 | 17 | 5 |
| 10% SPEEK-PSf/NMP | 83 | 17 | 10 |
| Feed Composition | Feed Concentration |
|---|---|
| NaCl | 1 mM, 5 mM |
| KCl | 1 mM, 5 mM |
| CaCl2 | 1 mM, 5 mM |
| BSA Protein | 100 ppm |
| PSf/NMP | 5%SPEEK-PSf/NMP | 10%SPEEK-PSf/NMP | ||||
|---|---|---|---|---|---|---|
| Name | PeakBE | Atomic% | PeakBE | Atomic% | PeakBE | Atomic% |
| C1s | 284.92 | 75.71 | 284.3 | 81.61 | 286.88 | 76.66 |
| O1s | 532.15 | 22.35 | 532 | 15.38 | 534.27 | 21.15 |
| S2p | 167.91 | 1.95 | 167.8 | 3.01 | 169.87 | 2.18 |
| Membrane | IEC | SD |
|---|---|---|
| PSf/NMP | 0.200286 | 0.05922 |
| 5% SPEEK-PSf/NMP | 0.608485 | 0.1842 |
| 10% SPEEK-PSf/NMP | 0.4186 | 0.1247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fionah, A.; McLarney, K.; Judd, A.; Escobar, I.C. Effects of the Applied Potential on the Performance of Polysulfone Membranes Functionalized with Sulfonated Polyether Ether Ketone Polymers. Membranes 2023, 13, 675. https://doi.org/10.3390/membranes13070675
Fionah A, McLarney K, Judd A, Escobar IC. Effects of the Applied Potential on the Performance of Polysulfone Membranes Functionalized with Sulfonated Polyether Ether Ketone Polymers. Membranes. 2023; 13(7):675. https://doi.org/10.3390/membranes13070675
Chicago/Turabian StyleFionah, Abelline, Kate McLarney, Aviana Judd, and Isabel C. Escobar. 2023. "Effects of the Applied Potential on the Performance of Polysulfone Membranes Functionalized with Sulfonated Polyether Ether Ketone Polymers" Membranes 13, no. 7: 675. https://doi.org/10.3390/membranes13070675