One-Step Hydrothermal Strategy for Preparation of a Self-Cleaning TiO2/SiO2 Fiber Membrane toward Oil-Water Separation in a Complex Environment
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Preparation of TSFM
2.3. Oil-Water Separation Tests
2.4. Mechanical Stability Tests
2.5. Environmental Stability Tests
2.6. Anti-Fouling and Self-Cleaning Performance Tests
2.7. Characterizations
3. Results and Discussion
3.1. Characterization of the Membranes
3.2. Surface Wettability of Membranes
3.3. Oil-Water Separation Performance
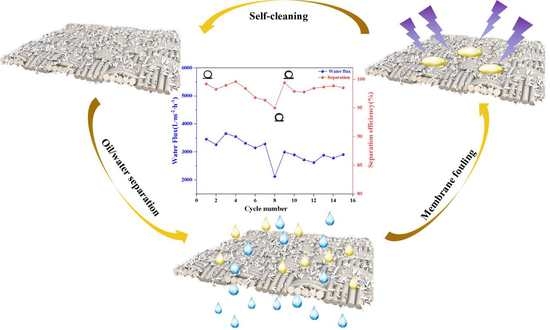
3.4. Stability and Anti-Fouling of Membranes
3.5. Separation Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- He, H.; Li, Z.; Ouyang, L.; Liang, Y.; Yuan, S. Hierarchical WO3@Cu(OH)2 nanorod arrays grown on copper mesh with superwetting and self-cleaning properties for high-performance oil/water separation. J. Alloys Compd. 2021, 855, 157421. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Easily enlarged and coating-free underwater superoleophobic fabric for oil/water and emulsion separation via a facile NaClO2 treatment. Sep. Purif. Technol. 2018, 195, 358–366. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.; Yang, T.; Bennett, P.; Zheng, Z.; Yang, Q.; Liu, D. Laser-structured superhydrophobic/superoleophilic aluminum surfaces for efficient oil/water separation. Environ. Sci. Pollut. Res. 2020, 27, 43138–43149. [Google Scholar] [CrossRef] [PubMed]
- Bakke, T.; Klungsoyr, J.; Sanni, S. Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry. Mar. Environ. Res. 2013, 92, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Seddighi, M.; Hejazi, S.M. Water-oil separation performance of technical textiles used for marine pollution disasters. Mar. Pollut. Bull. 2015, 96, 286–293. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Cai, X.; Han, Y.; Xiong, S. Oil–water pre-separation with a novel axial hydrocyclone. Chin. J. Chem. Eng. 2018, 26, 60–66. [Google Scholar] [CrossRef]
- Saththasivam, J.; Loganathan, K.; Sarp, S. An overview of oil-water separation using gas flotation systems. Chemosphere 2016, 144, 671–680. [Google Scholar] [CrossRef]
- Yang, D.; Feng, Y.; Wang, B.; Liu, Y.; Zheng, Y.; Sun, X.; Peng, J.; Feng, M.; Wang, D. An asymmetric AC electric field of triboelectric nanogenerator for efficient water/oil emulsion separation. Nano Energy 2021, 90, 106641. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Kurnia, K.A.; Siagian, U.W.R.; Ismadji, S.; Wenten, I.G. Membrane fouling and fouling mitigation in oil–water separation: A review. J. Environ. Chem. Eng. 2022, 10, 107532. [Google Scholar] [CrossRef]
- Fan, L.; Yan, J.; He, H.; Deng, N.; Zhao, Y.; Kang, W.; Cheng, B. Electro-blown spun PS/PAN fibrous membrane for highly efficient oil/water separation. Fibers Polym. 2017, 18, 1988–1994. [Google Scholar] [CrossRef]
- Guo, Y.; Li, M.; Wen, X.; Guo, X.; Zhang, T. Silica-modified electrospun membrane with underwater superoleophobicity for effective gravity-driven oil/water separation. Fibers Polym. 2022, 23, 1906–1914. [Google Scholar] [CrossRef]
- Shen, B.; Du, C.; Wang, W.; Yu, D. Antifouling hydrophilic electrostatic spinning PAN membrane based on click chemistry with high efficiency oil-water separation. Fibers Polym. 2022, 23, 3386–3397. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Liu, Y.; Xue, C.; Wang, Y.; Dai, J. Preparation of Janus membrane based on biomimetic polydopamine interface regulation and superhydrophobic attapulgite spraying for on-demand oil-water emulsion separation. J. Membr. Sci. 2021, 627, 119242. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Y.; Pan, Y.; Li, J.; Xie, A.; Xue, C.; Pan, J. Preparation of antifouling zwitterion PVDF-imprinted composite membranes for selective antibiotic and oil/water emulsion separation. React. Funct. Polym. 2023, 187, 105580. [Google Scholar] [CrossRef]
- Deng, W.; Li, C.; Pan, F.; Li, Y. Efficient oil/water separation by a durable underwater superoleophobic mesh membrane with TiO2 coating via biomineralization. Sep. Purif. Technol. 2019, 222, 35–44. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Lan, W.; Zhang, A.; Liu, C. Fabrication of sugarcane bagasse ester-based porous nanofiber membrane by electrospinning for efficient oil-water separation. Ind. Crop. Prod. 2022, 187, 115480. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, J.; Li, Y.; Zhang, X.; An, A.K.; Wang, Z. Superhydrophobic and superoleophilic PH-CNT membrane for emulsified oil-water separation. Desalination 2022, 526, 115536. [Google Scholar] [CrossRef]
- Xie, A.; Wu, Y.; Liu, Y.; Xue, C.; Ding, G.; Cheng, G.; Cui, J.; Pan, J. Robust antifouling NH2-MIL-88B coated quartz fibrous membrane for efficient gravity-driven oil-water emulsion separation. J. Membr. Sci. 2022, 644, 120093. [Google Scholar] [CrossRef]
- Xu, J.; Cui, J.; Sun, H.; Wu, Y.; Xue, C.; Xie, A.; Li, C. Facile preparation of hydrophilic PVDF membrane via tea polyphenols modification for efficient oil-water emulsion separation. Colloid Surf. A 2023, 657, 130639. [Google Scholar] [CrossRef]
- Helali, N.; Rastgar, M.; Farhad Ismail, M.; Sadrzadeh, M. Development of underwater superoleophobic polyamide-imide (PAI) microfiltration membranes for oil/water emulsion separation. Sep. Purif. Technol. 2020, 238, 116451. [Google Scholar] [CrossRef]
- Fan, J.B.; Song, Y.; Wang, S.; Meng, J.; Yang, G.; Guo, X.; Feng, L.; Jiang, L. Directly coating hydrogel on filter paper for effective oil-water separation in highly acidic, alkaline, and salty environment. Adv. Funct. Mater. 2015, 25, 5368–5375. [Google Scholar] [CrossRef]
- Wei, W.; Sun, M.; Zhang, L.; Zhao, S.; Wu, J.; Wang, J. Underwater oleophobic PTFE membrane for efficient and reusable emulsion separation and the influence of surface wettability and pore size. Sep. Purif. Technol. 2017, 189, 32–39. [Google Scholar] [CrossRef]
- Cui, J.; Xie, A.; Yan, Z.; Yan, Y. Fabrication of crosslinking modified PVDF/GO membrane with acid, alkali and salt resistance for efficient oil-water emulsion separation. Sep. Purif. Technol. 2021, 265, 118528. [Google Scholar] [CrossRef]
- Kallem, P.; Pandey, R.P.; Hegab, H.M.; Gaur, R.; Hasan, S.W.; Banat, F. High-performance thin-film composite forward osmosis membranes with hydrophilic PDA@TiO2 nanocomposite substrate for the treatment of oily wastewater under PRO mode. J. Environ. Chem. Eng. 2022, 10, 107454. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, F.; Wen, Q.; Yang, F.; Guo, Z. A novel polyacrylonitrile membrane with a high flux for emulsified oil/water separation. Sep. Purif. Technol. 2017, 184, 72–78. [Google Scholar] [CrossRef]
- Tai, M.H.; Gao, P.; Tan, B.Y.L.; Sun, D.D.; Leckie, J.O. Highly efficient and flexible electrospun carbon–silica nanofibrous membrane for ultrafast gravity-driven oil–water separation. ACS Appl. Mater. Interfaces 2014, 6, 9393–9401. [Google Scholar] [CrossRef]
- Yan, J.; Xiao, C.; Wang, C. Robust preparation of braid-reinforced hollow fiber membrane covered by PVDF nanofibers and PVDF/SiO2 micro/nanospheres for highly efficient emulsion separation. Sep. Purif. Technol. 2022, 298, 121593. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, Q.; Wang, Y.; Wang, J.; Meng, G. Separation of stable oil–water emulsion by the hydrophilic nano-sized ZrO2 modified Al2O3 microfiltration membrane. Sep. Purif. Technol. 2010, 75, 243–248. [Google Scholar] [CrossRef]
- Huang, A.; Chen, L.H.; Kan, C.C.; Hsu, T.Y.; Wu, S.E.; Jana, K.K.; Tung, K.L. Fabrication of zinc oxide nanostructure coated membranes for efficient oil/water separation. J. Membr. Sci. 2018, 566, 249–257. [Google Scholar] [CrossRef]
- Zou, D.; Kim, H.W.; Jeon, S.M.; Lee, Y.M. Robust PVDF/PSF hollow-fiber membranes modified with inorganic TiO2 particles for enhanced oil-water separation. J. Membr. Sci. 2022, 652, 120470. [Google Scholar] [CrossRef]
- Gondal, M.A.; Sadullah, M.S.; Qahtan, T.F.; Dastageer, M.A.; Baig, U.; McKinley, G.H. Fabrication and wettability study of WO3 coated photocatalytic membrane for oil-water separation: A comparative study with ZnO coated membrane. Sci. Rep. 2017, 7, 1686. [Google Scholar] [CrossRef]
- Li, Z.; He, H.; Liang, Y.; Ouyang, L.; Zhang, T.C.; Yuan, S. Photocatalytically driven self-cleaning and underwater superoleophobic copper mesh modified with hierarchical Bi2WO6@CuO nanowires for oil/water separation. Ind. Eng. Chem. Res. 2020, 59, 16450–16461. [Google Scholar] [CrossRef]
- Liu, Q.; Basel, N.; Li, L.; Xu, N.; Dong, Q.; Fan, L.; Wang, Q.; Ding, A.; Wang, T. Interfacial polymerization of a covalent organic framework layer on titanium dioxide@graphene oxide/polyacrylonitrile mixed-matrix membranes for high-performance dye separation. J. Membr. Sci. 2022, 647, 120296. [Google Scholar] [CrossRef]
- Kang, H.; Cheng, Z.; Lai, H.; Ma, H.; Liu, Y.; Mai, X.; Wang, Y.; Shao, Q.; Xiang, L.; Guo, X.; et al. Superlyophobic anti-corrosive and self-cleaning titania robust mesh membrane with enhanced oil/water separation. Sep. Purif. Technol. 2018, 201, 193–204. [Google Scholar] [CrossRef]
- Li, F.; Kong, W.; Zhao, X.; Pan, Y. Multifunctional TiO2-based superoleophobic/superhydrophilic coating for oil-water separation and oil purification. ACS Appl. Mater. Interfaces 2020, 12, 18074–18083. [Google Scholar] [CrossRef]
- Pi, J.K.; Yang, H.C.; Wan, L.S.; Wu, J.; Xu, Z.K. Polypropylene microfiltration membranes modified with TiO2 nanoparticles for surface wettability and antifouling property. J. Membr. Sci. 2016, 500, 8–15. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Gan, Z.Q.; Bao, R.Y.; Ke, K.; Liu, Z.Y.; Yang, M.B.; Yang, W. Green and robust superhydrophilic electrospun stereocomplex polylactide membranes: Multifunctional oil/water separation and self-cleaning. J. Membr. Sci. 2020, 593, 117420. [Google Scholar] [CrossRef]
- Nakamoto, W.; Hayami, R.; Aizawa, S.; Miyase, Y.; Fujii, S.; Yamamoto, K.; Gunji, T. Characterization of a flexible self-cleaning film with photoinduced hydrophilicity comprising phosphonic-acid-modified polysilsesquioxane-anchored titanium dioxide. Thin Solid Films 2020, 714, 138395. [Google Scholar] [CrossRef]
- Salehian, S.; Mehdipour, M.H.; Fotovat, F.; Mousavi, S.A. Photocatalytic TiO2@MIL-88A (Fe)/polyacrylonitrile mixed matrix membranes: Characterization, anti-fouling properties, and performance on the removal of natural organic matter. Chemosphere 2022, 302, 134893. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Su, B.L. Titanium oxide nanotubes, nanofibers and nanowires. Colloid Surf. A 2004, 241, 173–183. [Google Scholar] [CrossRef]
- Seo, H.K.; Kim, G.S.; Ansari, S.G.; Kim, Y.S.; Shin, H.S.; Shim, K.H.; Suh, E.-K. A study on the structure/phase transformation of titanate nanotubes synthesized at various hydrothermal temperatures. Sol. Energy Mater. Sol. Cells 2008, 92, 1533–1539. [Google Scholar] [CrossRef]
- Ramadan, H.S.; Ali, R.A.M.; Mobarak, M.; Badawi, M.; Selim, A.Q.; Mohamed, E.A.; Bonilla-Petriciolet, A.; Seliem, M.K. One-step fabrication of a new outstanding rutile TiO2 nanoparticles/anthracite adsorbent: Modeling and physicochemical interpretations for malachite green removal. Chem. Eng. J. 2021, 426, 131890. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. TiO2 NPs assembled into a carbon nanofiber composite electrode by a one-step electrospinning process for supercapacitor applications. Polymers 2019, 11, 899. [Google Scholar] [CrossRef]
- Yang, Y.; Lai, Q.; Mahmud, S.; Lu, J.; Zhang, G.; Huang, Z.; Wu, Q.; Zeng, Q.; Huang, Y.; Lei, H.; et al. Potocatalytic antifouling membrane with dense nano-TiO2 coating for efficient oil-in-water emulsion separation and self-cleaning. J. Membr. Sci. 2022, 645, 120204. [Google Scholar] [CrossRef]
- Yu, H.; Lian, Z.; Xu, J.; Wan, Y.; Wang, Z.; Li, Y.; Yu, Z.; Weng, Z. Mechanically durable underwater superoleophobic surfaces based on hydrophilic bulk metals for oil/water separation. Appl. Surf. Sci. 2018, 437, 400–409. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Li, W.; Li, H.; She, H.; Zha, F. Facile fabrication of underwater superoleophobic SiO2 coated meshes for separation of polluted oils from corrosive and hot water. Sep. Purif. Technol. 2016, 168, 209–214. [Google Scholar] [CrossRef]
- Liu, N.; Lin, X.; Zhang, W.; Cao, Y.; Chen, Y.; Feng, L.; Wei, Y. A pure inorganic ZnO-Co3O4 overlapped membrane for efficient oil/water emulsions separation. Sci. Rep. 2015, 5, 9688. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Hegde, N.D.; Hirashima, H. Absorption and desorption of organic liquids in elastic superhydrophobic silica aerogels. J. Colloid Interface Sci. 2007, 305, 124–132. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Z.; Mai, Z.; Ma, Y.; Liu, B.; Jiang, L.; Zhu, D. A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew. Chem. Int. Ed. 2004, 43, 2012–2014. [Google Scholar] [CrossRef]
- Sam, E.K.; Ge, Y.; Liu, J.; Lv, X. Robust, self-healing, superhydrophobic fabric for efficient oil/water emulsion separation. Colloid Surf. A 2021, 625, 126860. [Google Scholar] [CrossRef]
- Du, Q.; Zhou, P.; Pan, Y.; Qu, X.; Liu, L.; Yu, H.; Hou, J. Influence of hydrophobicity and roughness on the wetting and flow resistance of water droplets on solid surface: A many-body dissipative particle dynamics study. Chem. Eng. Sci. 2022, 249, 117327. [Google Scholar] [CrossRef]
- Wei, Y.; Qi, H.; Gong, X.; Zhao, S. Specially wettable membranes for oil–water separation. Adv. Mater. Interfaces 2018, 5, 1800576. [Google Scholar] [CrossRef]











| Elements (at.%) | C | O | B | Si | Ti |
|---|---|---|---|---|---|
| SiO2 fiber membrane | 62.20 | 27.22 | 2.0 | 8.58 | / |
| TSFM | 42.28 | 35.82 | 4.80 | 1.55 | 15.55 |
| Liquids | The Viscosity of Oils (mPa s) | Density (g cm−3) | Surface Tension (mN m−1) |
|---|---|---|---|
| Liquid paraffin | 14.2–17.2 | 0.86–0.91 | 33.1 |
| n-hexane | 0.33 | 0.66 | 18.4 |
| Petroleum ether | 0.3 | 0.66 | 18.8 |
| dichloroethane | 0.84 | 1.245 | 32.2 |
| Water | 0.89 | 1 | 72.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Xie, A.; Xu, J.; Xue, C.; Cui, J.; Pan, J. One-Step Hydrothermal Strategy for Preparation of a Self-Cleaning TiO2/SiO2 Fiber Membrane toward Oil-Water Separation in a Complex Environment. Membranes 2023, 13, 514. https://doi.org/10.3390/membranes13050514
Lin Y, Xie A, Xu J, Xue C, Cui J, Pan J. One-Step Hydrothermal Strategy for Preparation of a Self-Cleaning TiO2/SiO2 Fiber Membrane toward Oil-Water Separation in a Complex Environment. Membranes. 2023; 13(5):514. https://doi.org/10.3390/membranes13050514
Chicago/Turabian StyleLin, Yinghao, Atian Xie, Jian Xu, Changguo Xue, Jiuyun Cui, and Jianming Pan. 2023. "One-Step Hydrothermal Strategy for Preparation of a Self-Cleaning TiO2/SiO2 Fiber Membrane toward Oil-Water Separation in a Complex Environment" Membranes 13, no. 5: 514. https://doi.org/10.3390/membranes13050514