Preparation of a CAB−GO/PES Mixed Matrix Ultrafiltration Membrane and Its Antifouling Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
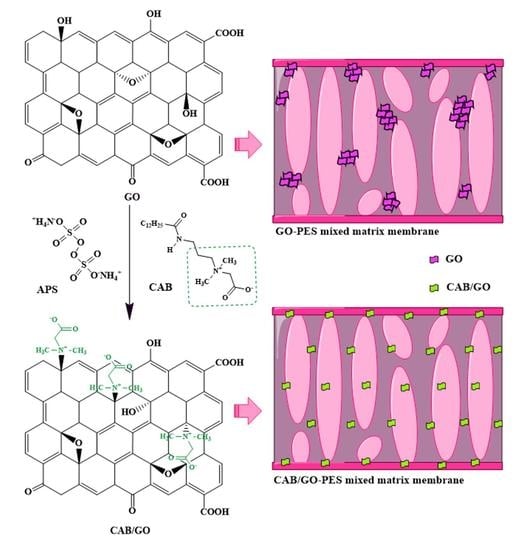
2.2. Synthesis of CAB−GO Nanosheets
2.3. Preparation of CAB−GO/PES Mixed Matrix Membranes
2.4. Characterization
2.5. Membrane Porosity and Average Pore Size
2.6. Permeation and Separation Performance Experiments
2.7. Mechanical Strength
2.8. Antifouling Performance
2.9. Effect of Transmembrane Pressure on Membrane Performance
3. Results and Discussion
3.1. Structural Characteristics of GO and CAB−GO
3.2. Structural Properties of CAB−GO/PES Mixed Matrix Membranes
3.3. Mechanical Properties
3.4. Hydrophilic Properties
3.5. Permeability and Separation Performance
3.6. Antifouling Performance and Stability
4. Conclusions
- (1)
- The dispersibility of GO grafted with CAB was obviously better than that of pristine GO, and the dispersion effect could be maintained for up to 24 h, indicating that CAB provided GO with sufficient long alkane chains, quaternary nitrogen atoms and amide groups. Due to its electrostatic interaction, the interlayer distance between CAB−GO nanosheets was increased, and the dispersibility of GO was improved to large extent, thereby effectively avoiding the phenomenon of GO agglomeration in organic solvents;
- (2)
- Based on the improvement of the surface porosity and surface hydrophilicity of the CAB−GO/PES mixed matrix membrane, the pure water flux of CGM-1.0 reached 461 L/(m2·h), which was 2.5 times higher than that of the original PES membrane. The CGM-0.1 also had a high pure water flux which was 180% higher than the original membrane. The rejection rates toward BSA and HA were above 96%;
- (3)
- Humic acid (HA) and bovine serum albumin (BSA) were used as target pollutants, the BSA rejection rate and corresponding HA rejection rate were increased from 87.46% to 96.57%, and from 88.64% to 97.70% after introducing CAB−GO, respectively. In the process of increasing the transmembrane pressure, CGM-0.1 exhibited better BSA (99.1%) and HA (98.1%) rejection at 1.5 bar.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- William, J.K.; Ryan, P.L. Water and beyond: Expanding the spectrum of large-scale energy efficient separation processes. AICHE J. 2012, 58, 2624–2633. [Google Scholar]
- Fane, A.G.; Wang, R.; Hu, M.X. Synthetic membranes for water purification: Status and future. Angew. Chem. Int. Edit. 2015, 54, 3368–3386. [Google Scholar] [CrossRef]
- Sheng, Z.; Zhang, J.; Liu, J. Liquid-based porous membranes. Chem. Soc. Rew. 2020, 49, 7907–7928. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface modification of water purification membranes. Angew. Chem. Int. Edit. 2017, 56, 4662–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.S.; Xue, J.M.; Ran, F.S.; Sun, D. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, G.E.; Sun, W.G.; Xu, Z.L.; Kong, Y.F.; Zheng, X.P.; Xu, S.J. Bio-inspired GO−Ag/PVDF/F127 membrane with improved anti-fouling for natural organic matter (NOM) resistance. Chem. Eng. J. 2017, 313, 450–460. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.E.; Wu, H.L.; Xu, Z.L.; Wan, J.J.; Liu, L.J.; Xu, S.J.; Kong, Y.F.; Wu, Q.; Min, J.; et al. Fabrication of GO−Ag/PVDF/F127 modified membrane IPA coagulation bath for catalytic reduction of 4-nitrophenol. Sep. Purif. Technol. 2020, 235, 116143. [Google Scholar] [CrossRef]
- Panacek, D.; Hochvaldova, L.; Bakandritsos, A.; Malina, T.; Langer, M.; Belza, J.; Martincova, J.; Vecerova, R.; Lazar, P.; Polakova, K.; et al. Silver covalently bound to cyanographene overcomes bacterial resistance to silver nanoparticles and antibiotics. Adv. Sci. 2021, 8, 2003090. [Google Scholar] [CrossRef]
- Cherkasov, A.N.; Tsareva, S.V.; Polotsky, A.E. Selective properties of ultrafiltration membranes from the standpoint of concentration polarization and adsorption phenomena. J. Membr. Sci. 1995, 104, 157–164. [Google Scholar] [CrossRef]
- Winter, J.; Barbeau, B.; Bérubé, P. Nanofiltration and Tight Ultrafiltration Membranes for Natural Organic Matter Removal—Contribution of Fouling and Concentration Polarization to Filtration Resistance. Membranes 2017, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.H.; Ye, S.J.; Zhang, G.; Li, W.B.; Gao, C.J.; Shen, C.; Meng, Q. Antimicrobial polysulfone blended ultrafiltration membranes prepared with Ag/Cu2O hybrid nanowires. J. Membr. Sci. 2016, 509, 83–93. [Google Scholar] [CrossRef]
- Racar, M.; Dolar, D.; Košutić, K. Chemical cleaning of flat sheet ultrafiltration membranes fouled by effluent organic matter. Separation and Purification Technology. Sep. Purif. Technol. 2017, 188, 140–146. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Q.; Biswas, P.; Fortner, J.D. Graphene oxides as nanofillers in polysulfone ultrafiltration membranes: Shape matters. J. Membr. Sci. 2019, 581, 453–461. [Google Scholar] [CrossRef]
- Chung, Y.T.; Mahmoudi, E.; Mohammad, A.W.; Benamor, A.; Johnson, D.; Hilal, N. Development of polysulfone-nanohybrid membranes using ZnO−GO composite for enhanced antifouling and antibacterial control. Desalination 2017, 402, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yan, B.; Hussain, Z.; Wei, W. Chemically graft aminated GO onto dehydro-fluorinated PVDF for preparation of homogenous DF−PVDF/GO−NH ultrafiltration membrane with high permeability and antifouling performance. Surf. Interfaces 2022, 33, 102255. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Zhang, L.; Meng, Q.; Shen, C.; Zhang, J. Novel polysulfone hybrid ultrafiltration membrane prepared with TiO2-g-HEMA and its antifouling characteristics. J. Membr. Sci. 2013, 436, 163–173. [Google Scholar] [CrossRef]
- Xu, H.; Ding, M.; Chen, W.; Li, Y.; Wang, K. Nitrogen-doped GO/TiO2 nanocomposite ultrafiltration membranes for improved photocatalytic performance. Sep. Purif. Technol. 2018, 195, 70–82. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Hou, J.; Zhang, Y.; Liu, J.; Bruggen, B.V. Graphene-based antimicrobial polymeric membranes: A review. J. Mater. Chem. A 2017, 5, 6776–6793. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, M.; Xu, Z.H.; Jiang, C.Y.; Shen, C.; Meng, Q. Guanidyl-functionalized graphene/polysulfone mixed matrix ultrafiltration membrane with superior permselective, antifouling and antibacterial properties for water treatment. J. Colloid Interface Sci. 2019, 540, 295–305. [Google Scholar] [CrossRef]
- Xu, C.; Liu, X.; Xie, B.; Yao, C.; Hu, W.; Li, Y.; Li, X. Preparation of PES ultrafiltration membranes with natural amino acids based zwitterionic antifouling surfaces. Appl. Surf. Sci. 2016, 385, 130–138. [Google Scholar] [CrossRef]
- Davenport, D.M.; Lee, J.; Elimelech, M. Efficacy of antifouling modification of ultrafiltration membranes by grafting zwitterionic polymer brushes. Sep. Purif. Technol. 2017, 189, 389–398. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.H.; Zhang, Y.F.; Zhang, X.; Meng, Q.; Zhu, Y.J.; Shen, C.; Lu, Y.H.; Zhang, G.L.; Gao, C.J. Confined assembly of ultrathin nanoporous nitrogen-doped graphene nanofilms with dual metal coordination chemistry. iScience 2021, 24, 102576. [Google Scholar] [CrossRef] [PubMed]
- Giaocabbo, A.; Bernardes, A.M.; Pinho, M.N. Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees. Sep. Purif. Technol. 2017, 173, 49–54. [Google Scholar] [CrossRef]
- Tseng, L.H.; Wang, P.H.; Li, W.C. Enhancing the ionic conductivity and mechanical properties of zwitterionic polymer electrolytes by betaine-functionalized graphene oxide for high-performance and flexible supercapacitors. J. Power Sources 2021, 516, 230624. [Google Scholar] [CrossRef]
- Ayyaru, S.; Ahn, Y.H. Application of sulfonic acid group functionalized graphene oxide to improve hydrophilicity, permeability, and antifouling of PVDF nanocomposite ultrafiltration membranes. J. Membr. Sci. 2017, 525, 210–219. [Google Scholar] [CrossRef]
- Giwa, A.; Hasan, S.W. Novel polyethersulfone-functionalized graphene oxide (PES−fGO) mixed matrix membranes for wastewater treatment. Sep. Purif. Technol. 2020, 241, 116735. [Google Scholar] [CrossRef]
- Liang, F.; Liu, D.; Dong, S. Facile construction of polyzwitterion membrane via assembly of graphene oxide-based core-brush nanosheet for high-efficiency water permeation. J. Membr. Sci. 2022, 644, 120150. [Google Scholar] [CrossRef]
- Li, J.; Zhu, W.; Zhang, S. Amine-terminated hyperbranched polyamide covalent functionalized graphene oxide-reinforced epoxy nanocomposites with enhanced toughness and mechanical properties. Polym. Test 2019, 76, 232–244. [Google Scholar] [CrossRef]
- Naeimi, H.; Shaabani, R. Preparation and characterization of functionalized graphene oxide Cu (I) complex: A facile and reusable nanocatalyst for microwave assisted heterocyclization of alkyl halides with alkynes and sodium azide. Catal. Commun. 2016, 87, 6–9. [Google Scholar] [CrossRef]
- Manafi, M.R.; Manafi, P.; Agarwal, S.; Bharti, A.K.; Asif, M.; Gupta, V.K. Synthesis of nanocomposites from polyacrylamide and graphene oxide: Application as flocculants for water purification. J. Colloid Interface Sci. 2017, 490, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.M.; Do Amparo, S.Z.; Lima, M.C. Microwave-assisted synthesis of polyacrylamide-aminated graphene oxide hybrid hydrogel with improved adsorption properties. J. Environ. Chem. Eng. 2020, 8, 104415. [Google Scholar] [CrossRef]
- Xu, Z.H.; Ye, X.W.; Hu, P.; Lv, B.S.; Zhang, G.L.; Meng, Q.; Gao, C.J. Azido-group functionalized graphene oxide/polysulfone mixed matrix ultrafiltration membrane with enhanced interfacial compatibility for efficient water and wastewater treatment. Sep. Purif. Technol. 2021, 283, 120162. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.W.; He, G.W.; Xing, R.S.; Pan, F.S.; Jiang, Z.Y.; Zhang, P.; Cao, X.Z.; Wang, B.Y. Incorporating zwitterionic graphene oxides into sodium alginate membrane for efficient water/alcohol separation. ACS Appl. Mater. Interfaces 2016, 8, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Zambere, R.S.; Dhopte, K.B.; Patwardhan, A.V. Polyamine functionalized graphene oxide polysulfone mixed matrix membranes with improved hydrophilicity and anti-fouling properties. Desalination 2017, 403, 24–35. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Xu, Z.H.; Zhang, T.T.; Meng, Q.; Zhang, X.; Shen, C.; Lu, Y.H.; Zhang, G.; Gao, C.J. Self-assembly of robust graphene oxide membranes with chirality for highly stable and selective molecular separation. J. Mater. Chem. A 2020, 8, 16985–16993. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, S.; Ma, C. D-spacing controllable GO membrane intercalated by sodium tetraborate pentahydrate for dye contamination wastewater treatment. J. Hazard. Mater. 2022, 422, 126939. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, H.; Meng, N. Graphene oxide incorporated thin film nanocomposite membrane at low concentration monomers. J. Membr. Sci. 2018, 565, 380–389. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, H.; Bernstein, R. Zwitterionic hydrogel modified reduced graphene oxide/ZnO nanocomposite blended membrane with high antifouling and antibiofouling performances. J. Colloid Interface Sci. 2022, 613, 426–434. [Google Scholar] [CrossRef]
- Cui, J.; Li, J.; Qiu, H. Zwitterionic graphene oxide modified with two silane molecules for multiple applications. Chem. Phys. Lett. 2018, 706, 543–547. [Google Scholar] [CrossRef]
- Liao, F.Q.; Xu, Z.H.; Fan, Z.X.; Meng, Q.; Lv, B.S.; Ye, X.W.; Shen, C.; Zhang, G.L. Confined assembly of ultrathin dual-functionalized Z-MXene nanosheets intercalated GO nanofilms with controlled structure for size-selective permeation. J. Mater. Chem. A. 2021, 9, 12236–12243. [Google Scholar] [CrossRef]
- Kallen, P.; Othman, I.; Ouad, M. Polyethersulfone hybrid ultrafiltration membranes fabricated with polydopamine modified ZnFe2O4 nanocomposites: Applications in humic acid removal and oil/water emulsion separation. Process Saf. Environ. 2021, 148, 813–824. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Faki, B. Flux-enhanced PVDF mixed matrix membranes incorporating APTS-functionalized graphene oxide for membrane distillation. J. Membr. Sci. 2018, 554, 309–323. [Google Scholar] [CrossRef]
- Wei, X.Z.; Yang, J.; Xu, Y.Y.; Shen, C.; Lu, Y.H.; Zhang, G.L.; Gao, C.J. Preparation and characterization of low-pressure nanofiltration membranes and the application in the separation process of dye molecules. J. Porous Mater. 2011, 9, 721–731. [Google Scholar] [CrossRef]
- Shi, Q.; Su, Y.; Chen, W. Grafting short-chain amino acids onto membrane surfaces to resist protein fouling. J. Membr. Sci. 2011, 366, 398–404. [Google Scholar] [CrossRef]
- Lau, S.K.; Yong, W.F. Recent progress of zwitterionic materials as antifouling membranes for ultrafiltration, nanofiltration, and reverse osmosis. ACS Appl. Polym. Mater. 2021, 3, 4390–4412. [Google Scholar] [CrossRef]












| Membrane | PES (wt%) | PVP (wt%) | GO (wt%) | Gly−GO (wt%) | CAB−GO (wt%) | DMAc (wt%) |
|---|---|---|---|---|---|---|
| CGM-0 | 16 | 0.1 | \ | \ | \ | 83.9 |
| GOM | 16 | 0.1 | 0.1 | \ | \ | 83.8 |
| GGM | 16 | 0.1 | \ | 0.1 | \ | 83.8 |
| CGM-0.05 | 16 | 0.1 | \ | \ | 0.05 | 83.85 |
| CGM-0.1 | 16 | 0.1 | \ | \ | 0.1 | 83.8 |
| CGM-0.3 | 16 | 0.1 | \ | \ | 0.3 | 83.6 |
| CGM-0.5 | 16 | 0.1 | \ | \ | 0.5 | 83.4 |
| CGM-1.0 | 16 | 0.1 | \ | \ | 1.0 | 82.9 |
| Membrane | Tensile Strength (MPa) | Elasticity Modulus (MPa) | Breaking Elongation (%) |
|---|---|---|---|
| CGM-0 | 1.43 (±0.38) | 68.61 (±11.29) | 6.40 (±0.03) |
| GOM | 1.76 (±0.27) | 81.61 (±21.60) | 4.20 (±0.39) |
| GGM | 1.99 (±0.15) | 103.14 (±5.34) | 4.24 (±0.16) |
| CGM-0.1 | 1.47 (±0.44) | 88.43 (±16.00) | 15.75 (±1.77) |
| CGM-0.3 | 1.93 (±0.53) | 83.08 (±8.33) | 17.02 (±4.85) |
| CGM-0.5 | 2.04 (±0.87) | 69.36 (±9.86) | 10.23 (±2.58) |
| CGM-1.0 | 0.96 (±0.44) | 65.18 (±8.20) | 14.96 (±4.75) |
| Membrane | Overall Porosity (%) | Mean Pore Size (nm) |
|---|---|---|
| CGM-0 | 45.58 (±1.53) | 8.15 (±0.34) |
| GOM | 47.93 (±1.69) | 9.75 (±0.63) |
| CGM-0.1 | 59.59 (±2.33) | 10.70 (±0.79) |
| CGM-0.3 | 62.68 (±1.47) | 13.55 (±0.51) |
| CGM-0.5 | 63.44 (±1.08) | 14.73 (±0.73) |
| CGM-1.0 | 63.00 (±1.52) | 14.90 (±0.58) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Wang, L.; Xu, W.; Xu, Z.; Zhang, G. Preparation of a CAB−GO/PES Mixed Matrix Ultrafiltration Membrane and Its Antifouling Performance. Membranes 2023, 13, 241. https://doi.org/10.3390/membranes13020241
Wu H, Wang L, Xu W, Xu Z, Zhang G. Preparation of a CAB−GO/PES Mixed Matrix Ultrafiltration Membrane and Its Antifouling Performance. Membranes. 2023; 13(2):241. https://doi.org/10.3390/membranes13020241
Chicago/Turabian StyleWu, Haiyan, Ling Wang, Wentao Xu, Zehai Xu, and Guoliang Zhang. 2023. "Preparation of a CAB−GO/PES Mixed Matrix Ultrafiltration Membrane and Its Antifouling Performance" Membranes 13, no. 2: 241. https://doi.org/10.3390/membranes13020241