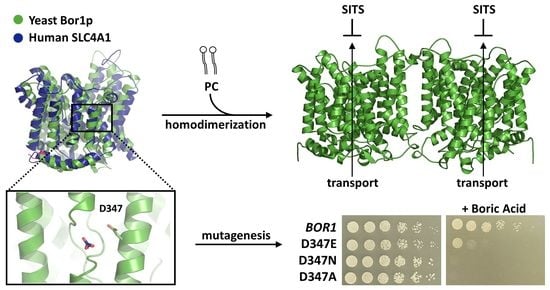
Borate Transporters and SLC4 Bicarbonate Transporters Share Key Functional Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Overexpression and Purification
2.2. Proteoliposome Reconstitution and Crosslinking Assays
2.3. Genetic Plating Assay
2.4. Borate Quantification Assay
2.5. Western Blot
3. Results and Discussion
3.1. A Dispensable N-Terminal Tail and a Conserved Functional Membrane Transport Fold
3.2. Lipids Promote ScBor1p Dimerization
3.3. Sensitivity to Derivatives of Stilbene Disulfonate
3.4. Identifying Functional Amino Acids at the Solute Binding Site
3.5. Disease-Causing Mutations from SLC4A1 Also Eliminate ScBor1p Function
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fairbanks, G.; Steck, T.L.; Wallach, D.F. Electrophoretic Analysis of the Major Polypeptides of the Human Erythrocyte Membrane. Biochemistry 1971, 10, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Reithmeier, R.A.F.; Casey, J.R.; Kalli, A.C.; Sansom, M.S.P.; Alguel, Y.; Iwata, S. Band 3, the Human Red Cell Chloride/Bicarbonate Anion Exchanger (AE1, SLC4A1), in a Structural Context. Biochim. Biophys. Acta 2016, 1858, 1507–1532. [Google Scholar] [CrossRef] [PubMed]
- Thurtle-Schmidt, B.H.; Stroud, R.M. Structure of Bor1 Supports an Elevator Transport Mechanism for SLC4 Anion Exchangers. Proc. Natl. Acad. Sci. USA 2016, 113, 10542–10546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coudray, N.; Seyler, S.L.; Lasala, R.; Zhang, Z.; Clark, K.M.; Dumont, M.E.; Rohou, A.; Beckstein, O.; Stokes, D.L. Structure of the SLC4 Transporter Bor1p in an Inward-Facing Conformation. Protein Sci. 2017, 26, 130–145. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, K.; Yasumori, M.; Imai, T.; Naito, S.; Matsunaga, T.; Oda, H.; Hayashi, H.; Chino, M.; Fujiwara, T. Bor1-1, an Arabidopsis Thaliana Mutant That Requires a High Level of Boron. Plant Physiol. 1997, 115, 901–906. [Google Scholar] [CrossRef] [Green Version]
- Takano, J.; Noguchi, K.; Yasumori, M.; Kobayashi, M.; Gajdos, Z.; Miwa, K.; Hayashi, H.; Yoneyama, T.; Fujiwara, T. Arabidopsis Boron Transporter for Xylem Loading. Nature 2002, 420, 337–340. [Google Scholar] [CrossRef]
- Kobayashi, M.; Matoh, T.; Azuma, J. Two Chains of Rhamnogalacturonan II Are Cross-Linked by Borate-Diol Ester Bonds in Higher Plant Cell Walls. Plant Physiol. 1996, 110, 1017–1020. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, M.A.; Eberhard, S.; Albersheim, P.; Darvill, A.G. Requirement of Borate Cross-Linking of Cell Wall Rhamnogalacturonan II for Arabidopsis Growth. Science 2001, 294, 846–849. [Google Scholar] [CrossRef]
- Takano, J.; Miwa, K.; Yuan, L.; von Wirén, N.; Fujiwara, T. Endocytosis and Degradation of BOR1, a Boron Transporter of Arabidopsis Thaliana, Regulated by Boron Availability. Proc. Natl. Acad. Sci. USA 2005, 102, 12276–12281. [Google Scholar] [CrossRef] [Green Version]
- Takano, J.; Kobayashi, M.; Noda, Y.; Fujiwara, T. Saccharomyces Cerevisiae Bor1p Is a Boron Exporter and a Key Determinant of Boron Tolerance. FEMS Microbiol. Lett. 2007, 267, 230–235. [Google Scholar] [CrossRef]
- Saouros, S.; Mohan, T.C.; Cecchetti, C.; Lehmann, S.; Barrit, J.D.; Scull, N.J.; Simpson, P.; Alguel, Y.; Cameron, A.D.; Jones, A.M.E.; et al. Structural and Functional Insights into the Mechanism of Action of Plant Borate Transporters. Sci. Rep. 2021, 11, 12328. [Google Scholar] [CrossRef]
- Arakawa, T.; Kobayashi-Yurugi, T.; Alguel, Y.; Iwanari, H.; Hatae, H.; Iwata, M.; Abe, Y.; Hino, T.; Ikeda-Suno, C.; Kuma, H.; et al. Crystal Structure of the Anion Exchanger Domain of Human Erythrocyte Band 3. Science 2015, 350, 680–684. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Liu, S.; Zhou, Z.H. Structure, Dynamics and Assembly of the Ankyrin Complex on Human Red Blood Cell Membrane. Nat. Struct. Mol. Biol. 2022, 29, 698–705. [Google Scholar] [CrossRef]
- Vallese, F.; Kim, K.; Yen, L.Y.; Johnston, J.D.; Noble, A.J.; Calì, T.; Clarke, O.B. Architecture of the Human Erythrocyte Ankyrin-1 Complex. Nat. Struct. Mol. Biol. 2022, 29, 706–718. [Google Scholar] [CrossRef]
- Zhekova, H.R.; Jiang, J.; Wang, W.; Tsirulnikov, K.; Kayık, G.; Khan, H.M.; Azimov, R.; Abuladze, N.; Kao, L.; Newman, D.; et al. CryoEM Structures of Anion Exchanger 1 Capture Multiple States of Inward- and Outward-Facing Conformations. Commun. Biol. 2022, 5, 1372. [Google Scholar] [CrossRef]
- Huynh, K.W.; Jiang, J.; Abuladze, N.; Tsirulnikov, K.; Kao, L.; Shao, X.; Newman, D.; Azimov, R.; Pushkin, A.; Zhou, Z.H.; et al. CryoEM Structure of the Human SLC4A4 Sodium-Coupled Acid-Base Transporter NBCe1. Nat. Commun. 2018, 9, 900. [Google Scholar] [CrossRef]
- Wang, W.; Tsirulnikov, K.; Zhekova, H.R.; Kayık, G.; Khan, H.M.; Azimov, R.; Abuladze, N.; Kao, L.; Newman, D.; Noskov, S.Y.; et al. Cryo-EM Structure of the Sodium-Driven Chloride/Bicarbonate Exchanger NDCBE. Nat. Commun. 2021, 12, 5690. [Google Scholar] [CrossRef]
- Ficici, E.; Faraldo-Gómez, J.D.; Jennings, M.L.; Forrest, L.R. Asymmetry of Inverted-Topology Repeats in the AE1 Anion Exchanger Suggests an Elevator-like Mechanism. J. Gen. Physiol. 2017, 149, 1149–1164. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Li, S.; Jiang, Y.; Jiang, J.; Fan, H.; Lu, G.; Deng, D.; Dang, S.; Zhang, X.; Wang, J.; et al. Structure and Mechanism of the Uracil Transporter UraA. Nature 2011, 472, 243–246. [Google Scholar] [CrossRef]
- Yu, X.; Yang, G.; Yan, C.; Baylon, J.L.; Jiang, J.; Fan, H.; Lu, G.; Hasegawa, K.; Okumura, H.; Wang, T.; et al. Dimeric Structure of the Uracil:Proton Symporter UraA Provides Mechanistic Insights into the SLC4/23/26 Transporters. Cell Res. 2017, 27, 1020–1033. [Google Scholar] [CrossRef]
- Alguel, Y.; Amillis, S.; Leung, J.; Lambrinidis, G.; Capaldi, S.; Scull, N.J.; Craven, G.; Iwata, S.; Armstrong, A.; Mikros, E.; et al. Structure of Eukaryotic Purine/H(+) Symporter UapA Suggests a Role for Homodimerization in Transport Activity. Nat. Commun. 2016, 7, 11336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, J.; Elferich, J.; Dehghani-Ghahnaviyeh, S.; Zhao, Z.; Meadows, M.; von Gersdorff, H.; Tajkhorshid, E.; Gouaux, E. Molecular Mechanism of Prestin Electromotive Signal Amplification. Cell 2021, 184, 4669–4679.e13. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, B.; Zhang, X.; Huang, X.; Zhang, M.; Guo, H.; Chen, X.; Huang, F.; Chen, T.; Mi, H.; et al. Structural Mechanism of the Active Bicarbonate Transporter from Cyanobacteria. Nat. Plants 2019, 5, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Geertsma, E.R.; Chang, Y.-N.; Shaik, F.R.; Neldner, Y.; Pardon, E.; Steyaert, J.; Dutzler, R. Structure of a Prokaryotic Fumarate Transporter Reveals the Architecture of the SLC26 Family. Nat. Struct. Mol. Biol. 2015, 22, 803–808. [Google Scholar] [CrossRef]
- Flores, S.; Feld, A.P.; Thurtle-Schmidt, B.H. Expression, Solubilization, and Purification of Eukaryotic Borate Transporters. J. Vis. Exp. 2019, 145, e59166. [Google Scholar] [CrossRef] [Green Version]
- Beltran, J.L.; Bain, R.K.; Stiefel, M.J.; McDonnell, A.S.; Carr, N.N.; Thurtle-Schmidt, B.H. Human SLC4A11 Does Not Complement BOR1 or Support Borate Transport in Saccharomyces Cerevisiae. MicroPubl. Biol. 2022. [Google Scholar] [CrossRef]
- Leano, J.B.; Batarni, S.; Eriksen, J.; Juge, N.; Pak, J.E.; Kimura-Someya, T.; Robles-Colmenares, Y.; Moriyama, Y.; Stroud, R.M.; Edwards, R.H. Structures Suggest a Mechanism for Energy Coupling by a Family of Organic Anion Transporters. PLoS Biol. 2019, 17, e3000260. [Google Scholar] [CrossRef] [Green Version]
- Mohan, T.C.; Jones, A.M.E. Determination of Boron Content Using a Simple and Rapid Miniaturized Curcumin Assay. Bio-Protoc. 2018, 8, e2703. [Google Scholar] [CrossRef] [Green Version]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Bonhivers, M.; Carbrey, J.M.; Gould, S.J.; Agre, P. Aquaporins in Saccharomyces. Genetic and Functional Distinctions between Laboratory and Wild-Type Strains. J. Biol. Chem. 1998, 273, 27565–27572. [Google Scholar] [CrossRef]
- Kanki, T.; Young, M.T.; Sakaguchi, M.; Hamasaki, N.; Tanner, M.J.A. The N-Terminal Region of the Transmembrane Domain of Human Erythrocyte Band 3. Residues Critical for Membrane Insertion and Transport Activity. J. Biol. Chem. 2003, 278, 5564–5573. [Google Scholar] [CrossRef] [Green Version]
- Pyle, E.; Guo, C.; Hofmann, T.; Schmidt, C.; Ribiero, O.; Politis, A.; Byrne, B. Protein-Lipid Interactions Stabilize the Oligomeric State of BOR1p from Saccharomyces Cerevisiae. Anal. Chem. 2019, 91, 13071–13079. [Google Scholar] [CrossRef]
- Prasad, R. Lipids in the Structure and Function of Yeast Membrane. Adv. Lipid Res. 1985, 21, 187–242. [Google Scholar] [CrossRef]
- Pyle, E.; Kalli, A.C.; Amillis, S.; Hall, Z.; Lau, A.M.; Hanyaloglu, A.C.; Diallinas, G.; Byrne, B.; Politis, A. Structural Lipids Enable the Formation of Functional Oligomers of the Eukaryotic Purine Symporter UapA. Cell Chem. Biol. 2018, 25, 840–848.e4. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-N.; Jaumann, E.A.; Reichel, K.; Hartmann, J.; Oliver, D.; Hummer, G.; Joseph, B.; Geertsma, E.R. Structural Basis for Functional Interactions in Dimers of SLC26 Transporters. Nat. Commun. 2019, 10, 2032. [Google Scholar] [CrossRef] [Green Version]
- Cabantchik, Z.I.; Rothstein, A. The Nature of the Membrane Sites Controlling Anion Permeability of Human Red Blood Cells as Determined by Studies with Disulfonic Stilbene Derivatives. J. Membr. Biol. 1972, 10, 311–330. [Google Scholar] [CrossRef]
- Cabantchik, Z.I.; Rothstein, A. Membrane Proteins Related to Anion Permeability of Human Red Blood Cells. I. Localization of Disulfonic Stilbene Binding Sites in Proteins Involved in Permeation. J. Membr. Biol. 1974, 15, 207–226. [Google Scholar] [CrossRef]
- Zhao, R.; Reithmeier, R.A. Expression and Characterization of the Anion Transporter Homologue YNL275w in Saccharomyces Cerevisiae. Am. J. Physiol. Cell Physiol. 2001, 281, C33–C45. [Google Scholar] [CrossRef]
- Jennings, M.L.; Smith, J.S. Anion-Proton Cotransport through the Human Red Blood Cell Band 3 Protein. Role of Glutamate 681. J. Biol. Chem. 1992, 267, 13964–13971. [Google Scholar] [CrossRef]
- Chernova, M.N.; Jiang, L.; Crest, M.; Hand, M.; Vandorpe, D.H.; Strange, K.; Alper, S.L. Electrogenic Sulfate/Chloride Exchange in Xenopus Oocytes Mediated by Murine AE1 E699Q. J. Gen. Physiol. 1997, 109, 345–360. [Google Scholar] [CrossRef]
- Tang, X.B.; Fujinaga, J.; Kopito, R.; Casey, J.R. Topology of the Region Surrounding Glu681 of Human AE1 Protein, the Erythrocyte Anion Exchanger. J. Biol. Chem. 1998, 273, 22545–22553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capper, M.J.; Yang, S.; Stone, A.C.; Vatansever, S.; Zilberg, G.; Mathiharan, Y.K.; Habib, R.; Hutchinson, K.; Schlessinger, A.; Mezei, M.; et al. Substrate Binding and Inhibition of the Anion Exchanger 1 Transporter. bioRxiv 2022. [Google Scholar] [CrossRef]
- Jennings, M.L.; Howren, T.R.; Cui, J.; Winters, M.; Hannigan, R. Transport and Regulatory Characteristics of the Yeast Bicarbonate Transporter Homolog Bor1p. Am. J. Physiol. Cell Physiol. 2007, 293, C468–C476. [Google Scholar] [CrossRef] [PubMed]
- Guizouarn, H.; Borgese, F.; Gabillat, N.; Harrison, P.; Goede, J.S.; McMahon, C.; Stewart, G.W.; Bruce, L.J. South-East Asian Ovalocytosis and the Cryohydrocytosis Form of Hereditary Stomatocytosis Show Virtually Indistinguishable Cation Permeability Defects. Br. J. Haematol. 2011, 152, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, A.; De Falco, L.; Borgese, F.; Esposito, M.R.; Avvisati, R.A.; Izzo, P.; Piscopo, C.; Guizouarn, H.; Biondani, A.; Pantaleo, A.; et al. A Novel Erythroid Anion Exchange Variant (Gly796Arg) of Hereditary Stomatocytosis Associated with Dyserythropoiesis. Haematologica 2009, 94, 1049–1059. [Google Scholar] [CrossRef]
- Yawata, Y.; Kanzaki, A.; Yawata, A.; Doerfler, W.; Ozcan, R.; Eber, S.W. Characteristic Features of the Genotype and Phenotype of Hereditary Spherocytosis in the Japanese Population. Int. J. Hematol. 2000, 71, 118–135. [Google Scholar]
- Bruce, L.J.; Robinson, H.C.; Guizouarn, H.; Borgese, F.; Harrison, P.; King, M.-J.; Goede, J.S.; Coles, S.E.; Gore, D.M.; Lutz, H.U.; et al. Monovalent Cation Leaks in Human Red Cells Caused by Single Amino-Acid Substitutions in the Transport Domain of the Band 3 Chloride-Bicarbonate Exchanger, AE1. Nat. Genet. 2005, 37, 1258–1263. [Google Scholar] [CrossRef]
- Jarolim, P.; Rubin, H.L.; Brabec, V.; Chrobak, L.; Zolotarev, A.S.; Alper, S.L.; Brugnara, C.; Wichterle, H.; Palek, J. Mutations of Conserved Arginines in the Membrane Domain of Erythroid Band 3 Lead to a Decrease in Membrane-Associated Band 3 and to the Phenotype of Hereditary Spherocytosis. Blood 1995, 85, 634–640. [Google Scholar] [CrossRef]
- Park, M.; Li, Q.; Shcheynikov, N.; Zeng, W.; Muallem, S. NaBC1 Is a Ubiquitous Electrogenic Na+-Coupled Borate Transporter Essential for Cellular Boron Homeostasis and Cell Growth and Proliferation. Mol. Cell 2004, 16, 331–341. [Google Scholar] [CrossRef]
- Ogando, D.G.; Jalimarada, S.S.; Zhang, W.; Vithana, E.N.; Bonanno, J.A. SLC4A11 Is an EIPA-Sensitive Na(+) Permeable PHi Regulator. Am. J. Physiol. Cell Physiol. 2013, 305, C716–C727. [Google Scholar] [CrossRef] [Green Version]
- Kao, L.; Azimov, R.; Abuladze, N.; Newman, D.; Kurtz, I. Human SLC4A11-C Functions as a DIDS-Stimulatable H+(OH−) Permeation Pathway: Partial Correction of R109H Mutant Transport. Am. J. Physiol. Cell Physiol. 2015, 308, C176–C188. [Google Scholar] [CrossRef]
- Loganathan, S.K.; Schneider, H.-P.; Morgan, P.E.; Deitmer, J.W.; Casey, J.R. Functional Assessment of SLC4A11, an Integral Membrane Protein Mutated in Corneal Dystrophies. Am. J. Physiol. Cell Physiol. 2016, 311, C735–C748. [Google Scholar] [CrossRef] [Green Version]
- Kato, A.; Kimura, Y.; Kurita, Y.; Chang, M.-H.; Kasai, K.; Fujiwara, T.; Hirata, T.; Doi, H.; Hirose, S.; Romero, M.F. Seawater Fish Use an Electrogenic Boric Acid Transporter, Slc4a11A, for Boric Acid Excretion by the Kidney. J. Biol. Chem. 2022, 299, 102740. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltran, J.L.; McGrath, L.G.; Caruso, S.; Bain, R.K.; Hendrix, C.E.; Kamran, H.; Johnston, H.G.; Collings, R.M.; Henry, M.-C.N.; Abera, T.-A.L.; et al. Borate Transporters and SLC4 Bicarbonate Transporters Share Key Functional Properties. Membranes 2023, 13, 235. https://doi.org/10.3390/membranes13020235
Beltran JL, McGrath LG, Caruso S, Bain RK, Hendrix CE, Kamran H, Johnston HG, Collings RM, Henry M-CN, Abera T-AL, et al. Borate Transporters and SLC4 Bicarbonate Transporters Share Key Functional Properties. Membranes. 2023; 13(2):235. https://doi.org/10.3390/membranes13020235
Chicago/Turabian StyleBeltran, Jean L., Lila G. McGrath, Sophia Caruso, Richara K. Bain, Claire E. Hendrix, Hana Kamran, Hartlee G. Johnston, Rebecca M. Collings, Menkara-Chinua N. Henry, Tsega-Ab L. Abera, and et al. 2023. "Borate Transporters and SLC4 Bicarbonate Transporters Share Key Functional Properties" Membranes 13, no. 2: 235. https://doi.org/10.3390/membranes13020235