Evaluation of Different Capture Solutions for Ammonia Recovery in Suspended Gas Permeable Membrane Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Conditions
2.3. Analysis Methodology
2.4. Data Calculations
2.5. Statistical Analysis
2.6. Economic Analysis of the Different NH3 Capture Solutions
3. Results and Discussion
3.1. pH and Electrical Conductivity Evolution of NH3 Trapping Solutions
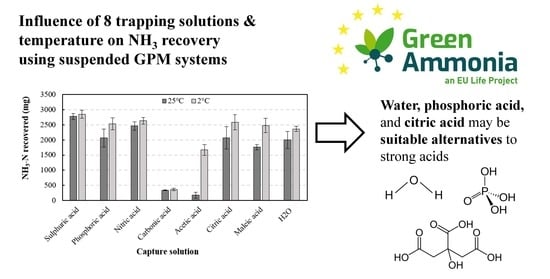
3.2. NH3-N Recovery Comparison at Different Temperatures
3.2.1. Evolution of NH3-N Recovery
3.2.2. NH3-N Recovery Comparison at 25 °C
3.2.3. NH3-N Recovery Comparison at 2 °C
3.2.4. Differences in NH3 Flux, NH3-N Capture, and NH3-N Removal Efficiencies
3.3. Comparison of Economic Costs Associated with the Different NH3 Capture Solutions on a Laboratory Scale
3.4. Applicability and Limitations of the Study
3.4.1. Applicability of the Study
3.4.2. Limitations of the Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanotti, M.B.; García-González, M.C.; Szögi, A.A.; Harrison, J.H.; Smith, W.B.; Moral, R. Removing and Recovering Nitrogen and Phosphorus from Animal Manure. Anim. Manure Prod. Charact. Environ. Concerns Manag. 2020, 67, 275–321. [Google Scholar] [CrossRef]
- Bouwman, L.; Goldewijk, K.K.; Van Der Hoek, K.W.; Beusen, A.H.W.; Van Vuuren, D.P.; Willems, J.; Rufino, M.C.; Stehfest, E. Erratum: Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl. Acad. Sci. USA 2013, 110, 21196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leip, A.; Billen, G.; Garnier, J.; Grizzetti, B.; Lassaletta, L.; Reis, S.; Simpson, D.; Sutton, M.A.; De Vries, W.; Weiss, F.; et al. Impacts of European livestock production: Nitrogen, sulphur, phosphorus and greenhouse gas emissions, land-use, water eutrophication and biodiversity. Environ. Res. Lett. 2015, 10, 115004. [Google Scholar] [CrossRef]
- Vanotti, M.; Szogi, A.; Pilar Bernal, M.; Martinez, J. Livestock waste treatment systems of the future: A challenge to environmental quality, food safety, and sustainability. OECD Workshop. Bioresour. Technol. 2009, 100, 5371–5373. [Google Scholar] [CrossRef] [Green Version]
- Vanotti, M.B.; Dube, P.J.; Szogi, A.A.; García-González, M.C. Recovery of ammonia and phosphate minerals from swine wastewater using gas-permeable membranes. Water Res. 2017, 112, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Bonmatí, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Shin, C.; Szczuka, A.; Jiang, R.; Mitch, W.A.; Criddle, C.S. Optimization of reverse osmosis operational conditions to maximize ammonia removal from the effluent of an anaerobic membrane bioreactor. Environ. Sci. Water Res. Technol. 2021, 7, 739–747. [Google Scholar] [CrossRef]
- Das, P.; Prasad, B.; Singh, K.K.K. Applicability of Zeolite Based Systems for Ammonia Removal and Recovery From Wastewater. Water Environ. Res. 2017, 89, 840–845. [Google Scholar] [CrossRef]
- Schwarzwälder Sprovieri, J.A.; Octavio de Souza, T.S.; Contrera, R.C. Ammonia removal and recovery from municipal landfill leachates by heating. J. Environ. Manag. 2020, 256, 109947. [Google Scholar] [CrossRef]
- Miles, A.; Ellis, T.G. Struvite precipitation potential for nutrient recovery from anaerobically treated wastes. Water Sci. Technol. 2001, 43, 259–266. [Google Scholar] [CrossRef]
- Riaño, B.; García-González, M.C. On-farm treatment of swine manure based on solid-liquid separation and biological nitrification-denitrification of the liquid fraction. J. Environ. Manag. 2014, 132, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Magrí, A.; Béline, F.; Dabert, P. Feasibility and interest of the anammox process as treatment alternative for anaerobic digester supernatants in manure processing—An overview. J. Environ. Manag. 2013, 131, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Gude, V.G. Wastewater treatment in microbial fuel cells—An overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Zhi, R.; Cao, K.; Zhang, G.; Zhu, J.; Xian, G. Zero excess sludge wastewater treatment with value-added substances recovery using photosynthetic bacteria. J. Clean. Prod. 2020, 250. [Google Scholar] [CrossRef]
- Garcia-González, M.C.; Vanotti, M.B. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of waste strength and pH. Waste Manag. 2015, 38, 455–461. [Google Scholar] [CrossRef]
- Lorick, D.; Macura, B.; Ahlström, M.; Grimvall, A.; Harder, R. Effectiveness of struvite precipitation and ammonia stripping for recovery of phosphorus and nitrogen from anaerobic digestate: A systematic review. Environ. Evid. 2020, 9, 1–20. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, Y.; Zhang, G.; Ruan, R.; Wang, Y.; Wu, X.; Zheng, H.; Zhang, Q.; Cao, L. New progress of ammonia recovery during ammonia nitrogen removal from various wastewaters. World J. Microbiol. Biotechnol. 2020, 36, 1–20. [Google Scholar] [CrossRef]
- Abeliovich, A.; Azov, Y. Toxicity of ammonia to algae in sewage oxidation ponds. Appl. Environ. Microbiol. 1976, 31, 801–806. [Google Scholar] [CrossRef] [Green Version]
- González-García, I.; Riaño, B.; Molinuevo-Salces, B.; Vanotti, M.B.; García-González, M.C. Improved anaerobic digestion of swine manure by simultaneous ammonia recovery using gas-permeable membranes. Water Res. 2021, 190. [Google Scholar] [CrossRef]
- Dube, P.J.; Vanotti, M.B.; Szogi, A.A.; García-González, M.C. Enhancing recovery of ammonia from swine manure anaerobic digester effluent using gas-permeable membrane technology. Waste Manag. 2016, 49, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Zuo, J.; Zhang, M.; Wang, Y.; Yu, H.; Li, B. Enhanced biogas production and in situ ammonia recovery from food waste using a gas-membrane absorption anaerobic reactor. Bioresour. Technol. 2019, 292, 121864. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe-Arachchige, S.P.; Cooke, P.; Nirmalakhandan, N. Recovery of nitrogen-fertilizer from centrate of anaerobically digested sewage sludge via gas-permeable membranes. J. Water Process Eng. 2020, 38, 101630. [Google Scholar] [CrossRef]
- Rothrock, M.J.; Szögi, A.A.; Vanotti, M.B. Recovery of ammonia from poultry litter using flat gas permeable membranes. Waste Manag. 2013, 33, 1531–1538. [Google Scholar] [CrossRef]
- Samani Majd, A.M.; Mukhtar, S. Ammonia recovery enhancement using a tubular gas-permeable membrane system in laboratory and field-scale studies. Trans. ASABE 2013, 56, 1951–1958. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Pilot plant for the capture of ammonia from the atmosphere of pig and poultry farms using gas-permeable membrane technology. Membranes 2021, 11, 859. [Google Scholar] [CrossRef]
- Tao, W.; Ukwuani, A.T. Coupling thermal stripping and acid absorption for ammonia recovery from dairy manure: Ammonia volatilization kinetics and effects of temperature, pH and dissolved solids content. Chem. Eng. J. 2015, 280, 188–196. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Evans, T.D. Recovering ammonium and struvite from digested sludge dewatering liquors. In Proceedings of the IWA Specialist Conference Moving Forward Wastewater Biosolids Sustainability, Jeju Island, Korea, 20–23 May 2007. [Google Scholar]
- Licon Bernal, E.E.; Maya, C.; Valderrama, C.; Cortina, J.L. Valorization of ammonia concentrates from treated urban wastewater using liquid-liquid membrane contactors. Chem. Eng. J. 2016, 302, 641–649. [Google Scholar] [CrossRef]
- Minocha, V.K.; Rao, A.V.S.P. Ammonia removal and recovery from urea fertilizer plant waste. Environ. Technol. Lett. 1988, 9, 655–664. [Google Scholar] [CrossRef]
- Damtie, M.M.; Volpin, F.; Yao, M.; Tijing, L.D.; Hailemariain, R.H.; Bao, T.; Park, K.D.; Shon, H.K.; Choi, J.S. Ammonia recovery from human urine as liquid fertilizers in hollow fiber membrane contactor: Effects of permeate chemistry. Environ. Eng. Res. 2021, 26, 1–9. [Google Scholar] [CrossRef]
- Jamaludin, Z.; Rollings-Scattergood, S.; Lutes, K.; Vaneeckhaute, C. Evaluation of sustainable scrubbing agents for ammonia recovery from anaerobic digestate. Bioresour. Technol. 2018, 270, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, F.M.; Povinelli, J.; Vieira, E.M. Ammonia removal from landfill leachate by air stripping and absorption. Environ. Technol. 2013, 34, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, J.; Waite, T.D. Ammonia-Rich Solution Production from Wastewaters Using Chemical-Free Flow-Electrode Capacitive Deionization. ACS Sustain. Chem. Eng. 2019, 7, 6480–6485. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Vanotti, M.B.; Martín-Ramos, P. Effect of acid flow rate, membrane surface area, and capture solution on the effectiveness of suspended gpm systems to recover ammonia. Membranes 2021, 11, 538. [Google Scholar] [CrossRef] [PubMed]
- Sürmeli, R.; Bayrakdar, A.; Çalli, B. Removal and recovery of ammonia from chicken manure. Water Sci. Technol. 2017, 75, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.R.; Losada, E.; Besteiro, R.; Arango, T.; Velo, R.; Ortega, J.A.; Fernandez, M.D. Evolution of NH3 concentrations in weaner pig buildings based on setpoint temperature. Agronomy 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Salgado, I.G.; Guigui, C.; Sperandio, M. Transmembrane chemical absorption technology for ammonia recovery from wastewater: A critical review. Chem. Eng. J. 2022, 444, 136491. [Google Scholar] [CrossRef]
- Reig, M.; Vecino, X.; Gibert, O.; Valderrama, C.; Cortina, J.L. Study of the operational parameters in the hollow fibre liquid-liquid membrane contactors process for ammonia valorisation as liquid fertiliser. Sep. Purif. Technol. 2021, 255, 117768. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wilhelm, E.; Battino, R.; Wilcock, R.J. Low-Pressure Solubility of Gases in Liquid Water. Chem. Rev. 1977, 77, 219–262. [Google Scholar] [CrossRef]
- Starmans, D.A.J.; Melse, R.W. Alternatives for the Use of Sulphuric Acid in Air Scrubbers; Report 385; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2011. (In Dutch) [Google Scholar]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ma, J.; Waite, T.D. The impact of absorbents on ammonia recovery in a capacitive membrane stripping system. Chem. Eng. J. 2020, 382, 122851. [Google Scholar] [CrossRef]
- Palakodeti, A.; Azman, S.; Rossi, B.; Dewil, R.; Appels, L. A critical review of ammonia recovery from anaerobic digestate of organic wastes via stripping. Renew. Sustain. Energy Rev. 2021, 143, 110903. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Sanchez Bascones, M.; Millner, P.; Szogi, A.A.; Hashem, F. Development of treatment modules for capturing ammonia emmisions from poultry manures and recovering the nitrogen. In Proceedings of the IIV Symposium on Agricultural and Agroindustrial Waste Management, Rio de Janeiro, Brazil, 5–7 May 2015; pp. 7–10. [Google Scholar]
- Hales, J.M.; Drewes, D.R. Solubility of ammonia in water at low concentrations. Atmos. Environ. 1979, 13, 1133–1147. [Google Scholar] [CrossRef]
- Kurtén, T.; Torpo, L.; Sundberg, M.R.; Kerminen, V.M.; Vehkamäki, H.; Kulmala, M. Estimating the NH3:H2so4 ratio of nucleating clusters in atmospheric conditions using quantum chemical methods. Atmos. Chem. Phys. 2007, 7, 2765–2773. [Google Scholar] [CrossRef] [Green Version]
- Riaño, B.; Molinuevo-Salces, B.; Vanotti, M.B.; García-González, M.C. Ammonia recovery from digestate using gas-permeable membranes: A pilot-scale study. Environments 2021, 8, 133. [Google Scholar] [CrossRef]
- De Dobbelaere, A.; De Keulenaere, B.; De Mey, J.; Lebuf, V.; Meers, E.; Ryckaert, B.; Schollier, C.; Van Driessche, J. Small-Scale Anaerobic Digestion: Case Studies in Western Europe; Inagro: Rumberke, Belgium, 2015. [Google Scholar]
- Cao, Y.; Bai, Z.; Misselbrook, T.; Wang, X.; Ma, L. Ammonia emissions from different pig production scales and their temporal variations in the North China Plain. J. Air Waste Manag. Assoc. 2021, 71, 23–33. [Google Scholar] [CrossRef]
- Arogo, J.; Zhang, R.H.; Riskowski, G.L.; Christianson, L.L.; Day, D.L. Mass transfer coefficient of ammonia in liquid swine manure and aqueous solutions. J. Agric. Eng. Res. 1999, 73, 77–86. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, B.; Jia, Y.; He, F.; Chen, W. Mitigation Strategies of Air Pollutants for Mechanical Ventilated Livestock and Poultry Housing—A Review. Atmosphere 2022, 13, 452. [Google Scholar] [CrossRef]
- Brienza, C.; Sigurnjak, I.; Michels, E.; Meers, E. Ammonia Stripping and Scrubbing for Mineral Nitrogen Recovery. In Biorefinery of Inorganics: Recovering Mineral Nutrients from Biomass and Organic Waste; Meers, E., Velthof, G., Michels, E., Rietra, R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 95–106. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Paredes, C.; Zhang, H.; Giles, C.D.; Darch, T.; Stutter, M.; George, T.S.; Shand, C.; Lumsdon, D.; Cooper, P.; et al. Organic Acids Regulation of Chemical-Microbial Phosphorus Transformations in Soils. Environ. Sci. Technol. 2016, 50, 11521–11531. [Google Scholar] [CrossRef]



| Stripping Solution | Parameter | T (°C) | Experimental Time | |
|---|---|---|---|---|
| Day 1 | Day 7 | |||
| Sulfuric Acid | pH | 25 | 0.6 ± 0.0 | 0.7 ± 0.0 |
| 2 | 0.3 ± 0.1 | 0.5 ± 0.2 | ||
| CE | 25 | 207.3 ± 0.6 | 170.5 ± 3.1 | |
| 2 | 289.7 ± 19.3 | 206.7 ± 5.5 | ||
| Phosporic Acid | pH | 25 | 1.3 ± 0.1 | 2.0 ± 0.0 |
| 2 | 1.3 ± 0.1 | 2.0 ± 0.1 | ||
| CE | 25 | 23.2 ± 0.3 | 18.6 ± 0.4 | |
| 2 | 22.9 ± 0.6 | 18.2 ± 0.9 | ||
| Nitric acid | pH | 25 | 0.5 ± 0.0 | 0.6 ± 0.1 |
| 2 | 0.4 ± 0.0 | 0.6 ± 0.0 | ||
| CE | 25 | 224.7 ± 1.2 | 174.3 ± 14.5 | |
| 2 | 234.7 ± 1.2 | 181.2 ± 2.6 | ||
| Carbonic acid | pH | 25 | 6.0 ± 0.6 | 6.6 ± 0.0 |
| 2 | 5.9 ± 1.3 | 6.5 ± 0.1 | ||
| CE | 25 | 0.2 ± 0.0 | 4.4 ± 0.2 | |
| 2 | 0.3 ± 0.1 | 3.5 ± 0.3 | ||
| Acetic acid | pH | 25 | 2.4 ± 0.0 | 2.9 ± 0.2 |
| 2 | 2.1 ± 0.1 | 3.8 ± 0.0 | ||
| CE | 25 | 1.5 ± 0.1 | 1.9 ± 0.5 | |
| 2 | 1.6 ± 0.2 | 9.3 ± 0.6 | ||
| Citric acid | pH | 25 | 1.7 ± 0.1 | 3.0 ± 0.1 |
| 2 | 1.7 ± 0.1 | 3.0 ± 0.0 | ||
| CE | 25 | 5.8 ± 0.1 | 11.2 ± 1.0 | |
| 2 | 5.7 ± 0.1 | 12.3 ± 0.4 | ||
| Maleic acid | pH | 25 | 1.3 ± 0.0 | 1.5 ± 0.1 |
| 2 | 1.1 ± 0.0 | 1.7 ± 0.1 | ||
| CE | 25 | 31.9 ± 0.1 | 26.5 ± 0.1 | |
| 2 | 30.8 ± 0.3 | 25.2 ± 0.1 | ||
| H2O | pH | 25 | 6.7 ± 0.2 | 8.6 ± 0.1 |
| 2 | 6.0 ± 0.2 | 8.7 ± 0.0 | ||
| CE | 25 | 0.7 ± 0.1 | 12.4 ± 1.8 | |
| 2 | 0.4 ± 0.2 | 9.6 ± 0.1 | ||
| Capture Solution | T (°C) | N Flux (mg N·cm−2·d−1) | NH3-N Capture Efficiency (%) | NH3-N Removal Efficiency (%) |
|---|---|---|---|---|
| Sulfuric acid | 25 | 2.4 ± 0.1 a | 87 | 46 |
| 2 | 2.5 ± 0.1 a | 89 | 48 | |
| Phosphoric acid | 25 | 1.8 ± 0.3 bcde | 64 | 34 |
| 2 | 2.2 ± 0.2 abc | 79 | 42 | |
| Nitric acid | 25 | 2.2 ± 0.1 abc | 77 | 41 |
| 2 | 2.3 ± 0.1 ab | 82 | 44 | |
| Carbonic acid | 25 | 0.3 ± 0.0 f | 11 | 6 |
| 2 | 0.3 ± 0.0 f | 12 | 6 | |
| Acetic acid | 25 | 0.2 ± 0.1 f | 6 | 3 |
| 2 | 1.5 ± 0.2 e | 52 | 28 | |
| Citric acid | 25 | 1.8 ± 0.3 bcde | 65 | 34 |
| 2 | 2.3 ± 0.2 abc | 81 | 43 | |
| Maleic acid | 25 | 1.5 ± 0.1 de | 55 | 29 |
| 2 | 2.2 ± 0.2 abc | 77 | 41 | |
| H2O | 25 | 1.7 ± 0.3 cde | 62 | 33 |
| 2 | 2.1 ± 0.1 abcd | 74 | 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Evaluation of Different Capture Solutions for Ammonia Recovery in Suspended Gas Permeable Membrane Systems. Membranes 2022, 12, 572. https://doi.org/10.3390/membranes12060572
Soto-Herranz M, Sánchez-Báscones M, Antolín-Rodríguez JM, Martín-Ramos P. Evaluation of Different Capture Solutions for Ammonia Recovery in Suspended Gas Permeable Membrane Systems. Membranes. 2022; 12(6):572. https://doi.org/10.3390/membranes12060572
Chicago/Turabian StyleSoto-Herranz, María, Mercedes Sánchez-Báscones, Juan Manuel Antolín-Rodríguez, and Pablo Martín-Ramos. 2022. "Evaluation of Different Capture Solutions for Ammonia Recovery in Suspended Gas Permeable Membrane Systems" Membranes 12, no. 6: 572. https://doi.org/10.3390/membranes12060572