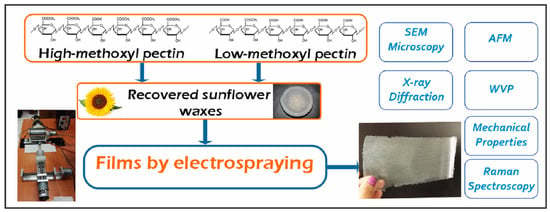
Pectin Films with Recovered Sunflower Waxes Produced by Electrospraying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Elaboration of Solutions and Emulsions
2.3. Density, Refractive Index and Surface Tension
2.4. Electric Conductivity (σ)
2.5. Viscosity
2.6. Electrosprying
2.7. Films Characterization
2.7.1. Film Thickness
2.7.2. Scanning Electron Microscopy (SEM)
2.7.3. Atomic Force Microscopy (AFM)
2.7.4. X-ray Diffraction
2.7.5. Water Vapor Permeability (WVP)
2.7.6. Mechanical Properties
2.7.7. Raman Spectroscopy
2.7.8. Statistical Analysis
3. Results and Discussion
3.1. Film-Forming Solutions Characterization
3.2. Films Characterization
3.3. SEM Microscopy
3.4. X-ray Diffraction
3.5. Atomic Force Microscopy (AFM)
3.6. Mechanical Properties
3.7. Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghanbarzadeh, B.; Oleyaei, S.A.; Almasi, H. Nanostructured materials utilized in biopolymer-based plastics for food packaging applications. Crit. Rev. Food Sci. Nutr. 2015, 55, 1699–1723. [Google Scholar] [CrossRef] [PubMed]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; Garrigos, M.d.C.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2016, 133, 42631. [Google Scholar] [CrossRef] [Green Version]
- Dash, K.K.; Ali, N.A.; Das, D.; Mohanta, D. Thorough evaluation of sweet potato starch and lemon-waste pectin based-edible films with nano-titania inclusions for food packaging applications. Int. J. Biol. Macromol. 2019, 139, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Janjarasskul, T.; Krochta, J.M. Edible packaging materials. Annu. Rev. Food Sci. Technol. 2010, 1, 415–448. [Google Scholar] [CrossRef] [PubMed]
- Debeaufort, F.; Voilley, A. Effect of surfactants and drying rate on barrier properties of emulsified edible films. International J. Food Sci. Technol. 1995, 30, 183–190. [Google Scholar] [CrossRef]
- Rai, S.K.; Chaturvedi, K.; Yadav, S.K. Evaluation of structural integrity and functionality of commercial pectin based edible films incorporated with corn flour, beetroot, orange peel, muesli and rice flour. Food Hydrocoll. 2019, 91, 127–135. [Google Scholar]
- Khan, M.K.I.; Nazir, A.; Maan, A.A. Electrospraying: A novel technique for efficient coating of foods. Food Eng. Rev. 2017, 9, 112–119. [Google Scholar] [CrossRef]
- Pavlath, A.E.; Orts, W. Edible films and coatings: Why, what, and how? In Edible Films and Coatings for Food Applications; Embuscado, M.E., Huber, K.C., Eds.; Springer: New York, NY, USA, 2009; pp. 1–23. [Google Scholar]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Chalapud, M.C.; Baümler, E.R.; Carelli, A.A. Edible films based on aqueous emulsions of low-methoxyl pectin with recovered and purified sunflower waxes. J. Sci. Food Agric. 2020, 100, 2675–2687. [Google Scholar] [CrossRef]
- Baümler, E.R.; Carelli, A.A.; Martini, S. Preparation and physical properties of calcium pectinate films modified with sunflower wax. J Eur. J. Lipid Sci. Technol. 2014, 116, 1534–1545. [Google Scholar] [CrossRef]
- Valdespino-León, M.; Calderón-Domínguez, G.; De La Paz Salgado-Cruz, M.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Morales-Sánchez, E.; Gaona-Sánchez, V.A.; Terrazas-Valencia, F. Biodegradable electrosprayed pectin films: An alternative to valorize coffee mucilage. Waste Biomass Valorization 2021, 12, 2477–2494. [Google Scholar] [CrossRef]
- Younis, H.G.; Abdellatif, H.R.; Ye, F.; Zhao, G. Tuning the physicochemical properties of apple pectin films by incorporating chitosan/pectin fiber. Int. J. Biol. Macromol. 2020, 159, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Natural antioxidants-based edible active food packaging: An overview of current advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Sood, A.; Saini, C. Red pomelo peel pectin based edible composite films: Effect of pectin incorporation on mechanical, structural, morphological and thermal properties of composite films. Food Hydrocoll. 2022, 123, 107135. [Google Scholar] [CrossRef]
- Pérez Espitia, P.J.; Du, W.-X.; Avena Bustillos, R.d.J.; Ferreira Soares, N.d.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Wang, J.; Dumas, E.; Gharsallaoui, A. Low Methoxyl pectin/sodium caseinate complexing behavior studied by isothermal titration calorimetry. Food Hydrocoll. 2019, 88, 163–169. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Fishman, M.L.; Hotchkiss Jr, A.T.; Lee, H.G. Viscometric behavior of high-methoxy and low-methoxy pectin solutions. Food Hydrocoll. 2006, 20, 62–67. [Google Scholar] [CrossRef]
- Sayah, M.Y.; Chabir, R.; Benyahia, H.; Rodi Kandri, Y.; Ouazzani Chahdi, F.; Touzani, H.; Errachidi, F. Yield, esterification degree and molecular weight evaluation of pectins isolated from orange and grapefruit peels under different conditions. PLoS ONE 2016, 11, e0161751. [Google Scholar] [CrossRef] [Green Version]
- Mendes, J.; Norcino, L.; Manrich, A.; Pinheiro, A.; Oliveira, J.; Mattoso, L. Characterization of pectin films integrated with cocoa butter by continuous casting: Physical, thermal and barrier properties. J. Polym. Environ. 2020, 28, 2905–2917. [Google Scholar] [CrossRef]
- Pérez, C.D.; De’Nobili, M.D.; Rizzo, S.A.; Gerschenson, L.N.; Descalzo, A.M.; Rojas, A. High methoxyl pectin–methyl cellulose films with antioxidant activity at a functional food interface. J. Food Eng. 2013, 116, 162–169. [Google Scholar] [CrossRef]
- Chaichi, M.; Hashemi, M.; Badii, F.; Mohammadi, A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr. Polym. 2017, 157, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Chetouani, A.; Elkolli, M.; Bounekhel, M.; Benachour, D. Synthesis and properties of novel hydrogels from oxidized pectin crosslinked gelatin for biomedical applications. Polym. Bull. 2014, 71, 2303–2316. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V. Edible bio-nano-hybrid coatings for food protection based on pectins and LDH-salicylate: Preparation and analysis of physical properties. LWT-Food Sci. Technol. 2016, 69, 139–145. [Google Scholar] [CrossRef]
- Ye, Y.; Zeng, F.; Zhang, M.; Zheng, S.; Li, J.; Fei, P. Hydrophobic edible composite packaging membrane based on low-methoxyl pectin/chitosan: Effects of lotus leaf cutin. Food Packag. Shelf Life 2020, 26, 100592. [Google Scholar] [CrossRef]
- Gohil, R.M. Synergistic blends of natural polymers, pectin and sodium alginate. J. Appl. Polym. Sci. 2011, 120, 2324–2336. [Google Scholar] [CrossRef]
- Bayarri, M.; Oulahal, N.; Degraeve, P.; Gharsallaoui, A. Properties of lysozyme/low methoxyl (LM) pectin complexes for antimicrobial edible food packaging. J. Food Eng. 2014, 131, 18–25. [Google Scholar] [CrossRef]
- Gaona Sánchez, V.A.; Calderón Domínguez, G.; Morales Sánchez, E.; Chanona Pérez, J.J.; Arzate Vázquez, I.; Terrés Rojas, E. Pectin-based films produced by electrospraying. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Brinques, G.B.; Siqueira, N.M.; Pletsch, J.; Soares, R.M.D.; Ayub, M.A.Z. Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. J. Funct. Foods 2016, 24, 316–326. [Google Scholar] [CrossRef]
- Gaona-Sánchez, V.A.; Calderón-Domínguez, G.; Morales-Sánchez, E.; Moreno-Ruiz, L.A.; Terrés-Rojas, E.; Salgado-Cruz, M.d.l.P.; Escamilla-García, M.; Barrios-Francisco, R. Physicochemical and superficial characterization of a bilayer film of zein and pectin obtained by electrospraying. J. Appl. Polym. Sci. 2021, 138, 50045. [Google Scholar] [CrossRef]
- Xu, C.; Ma, J.; Wang, W.; Liu, Z.; Gu, L.; Qian, S.; Hou, J.; Jiang, Z. Preparation of pectin-based nanofibers encapsulating Lactobacillus rhamnosus 1.0320 by electrospinning. Food Hydrocoll. 2022, 124, 107216. [Google Scholar] [CrossRef]
- Chalapud, M.C.; Baümler, E.R.; Carelli, A.A. Emulsions of sunflower wax in pectin aqueous solutions: Physical characterization and stability. Food Res. Int. 2018, 108, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Chalapud, M.C.; Baümler, E.R.; Carelli, A.A. Characterization of waxes and residual oil recovered from sunflower oil winterization waste. Eur. J. Lipid Sci. Technol. 2017, 119, 1500608. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Mujawar, L.H.; Schutyser, M.A.; Schroën, K.; Boom, R. Deposition of thin lipid films prepared by electrospraying. Food Bioprocess Technol. 2013, 6, 3047–3055. [Google Scholar] [CrossRef]
- Gaona Sánchez, V.A.; Calderon-Dominguez, G.; Morales-Sanchez, E.; Chanona-Perez, J.J.; Velazquez-De La Cruz, G.; Mendez-Mendez, J.V.; Terrés-Rojas, E.; Farrera-Rebollo, R.R. Preparation and characterisation of zein films obtained by electrospraying. Food Hydrocoll. 2015, 49, 1–10. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Schutyser, M.; Schroën, K.; Boom, R. Barrier properties and storage stability of edible coatings prepared with electrospraying. Innov. Food Sci. Emerg. Technol. 2014, 23, 182–187. [Google Scholar] [CrossRef]
- Antoniewski, M.N.; Barringer, S.; Knipe, C.; Zerby, H. Effect of a gelatin coating on the shelf life of fresh meat. J. Food Sci. 2007, 72, E382–E387. [Google Scholar] [CrossRef]
- Bravin, B.; Peressini, D.; Sensidoni, A. Development and application of polysaccharide–lipid edible coating to extend shelf-life of dry bakery products. J. Food Eng. 2006, 76, 280–290. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.-X.; Avena-Bustillos, R.J.; Berrios, J.D.J.; Sambo, P.; McHugh, T.H. Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess Technol. 2017, 10, 165–174. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemist: Gaithersburg, MA, USA, 2000. [Google Scholar]
- Calixto-Rodríguez, M.; Sánchez-Juárez, A. Películas delgadas de SnS2 preparadas por la técnica de Rocío Pirolítico. Superf. Y Vacío 2007, 20, 34–38. [Google Scholar]
- Ribotta, P.D.; Cuffini, S.; León, A.E.; Añón, M.C. The staling of bread: An X-ray diffraction study. Eur. Food Res. Technol. 2004, 218, 219–223. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M.; Velazquez, G. Novel composite films based on cellulose reinforced with chitosan and polyvinyl alcohol: Effect on mechanical properties and water vapour permeability. Polym. Test. 2018, 69, 536–544. [Google Scholar] [CrossRef]
- ASTM Standard E96/E96M-05; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2005.
- ASTM Standard D882-09; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2009.
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Yapo, B.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Luzio, G.A.; Cameron, R. Contactless conductivity: An HPLC method to analyze degree of methylation of pectin. Proc. Fla. State Hortic. Soc. 2010, 123, 213–216. [Google Scholar]
- Morris, G.A.; de al Torre, J.G.; Ortega, A.; Castile, J.; Smith, A.; Harding, S.E. Molecular flexibility of citrus pectins by combined sedimentation and viscosity analysis. Food Hydrocoll. 2008, 22, 1435–1442. [Google Scholar] [CrossRef] [Green Version]
- Cha, D.S.; Chinnan, M.S. Biopolymer-based antimicrobial packaging: A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar] [CrossRef]
- Morillon, V.; Debeaufort, F.; Blond, G.; Capelle, M.; Voilley, A. Factors affecting the moisture permeability of lipid-based edible films: A review. Crit. Rev. Food Sci. Nutr. 2002, 42, 67–89. [Google Scholar] [CrossRef]
- Debeaufort, F.; Voilley, A. Lipid-based edible films and coatings. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 135–168. [Google Scholar]
- Jooyandeh, H. Whey protein films and coatings: A review. Pak. J. Nutr. 2011, 10, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Wongphan, P.; Panrong, T.; Harnkarnsujarit, N. Effect of different modified starches on physical, morphological, thermomechanical, barrier and biodegradation properties of cassava starch and polybutylene adipate terephthalate blend film. Food Packag. Shelf Life 2022, 32, 100844. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qian, L. Enhanced water vapour barrier and grease resistance of paper bilayer-coated with chitosan and beeswax. Carbohydr. Polym. 2014, 101, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, N.; Mueller, M.; Nasui, L.; Schmid, M. Effects of film constituents on packaging-relevant properties of sodium caseinate-based emulsion films. Prog. Org. Coat. 2018, 114, 250–258. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Tensile properties and water vapor permeability of sodium caseinate films containing oleic acid–beeswax mixtures. J. Food Eng. 2008, 85, 393–400. [Google Scholar] [CrossRef]
- Martin-Polo, M.; Mauguin, C.; Voilley, A. Hydrophobic films and their efficiency against moisture transfer. 1. Influence of the film preparation technique. J. Agric. Food Chem. 1992, 40, 407–412. [Google Scholar] [CrossRef]
- Giancone, T.; Torrieri, E.; Di Pierro, P.; Cavella, S.; Giosafatto, C.V.; Masi, P. Effect of surface density on the engineering properties of high methoxyl pectin-based edible films. Food Bioprocess Technol. 2011, 4, 1228–1236. [Google Scholar] [CrossRef] [Green Version]
- Oakenfull, D. The chemistry of high-methoxyl pectins. In The Chemistry and Technology of Pectin, Walter R.H., Ed.; Academic Press, Inc.: San Diego, CA, USA, 1991; pp. 87–108. [Google Scholar]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly (Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef]
- De Carvalho Faria, M.A.; da Silva Sousa, M.; Dos Santos, K.F.; de Souza, N.C.; Silva, J.R. Preparation and characterization of epicuticular wax films. Heliyon 2019, 5, e01319. [Google Scholar] [CrossRef] [Green Version]
- Mal, B.; Ray, S.; Shamanna, J. Surface properties and scaling behavior of a generalized ballistic deposition model. Phys. Rev. E 2016, 93, 022121. [Google Scholar] [CrossRef] [Green Version]
- Galus, S.; Turska, A.; Lenart, A. Sorption and wetting properties of pectin edible films. Czech J. Food Sci. 2012, 30, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Meerasri, J.; Sothornvit, R. Characterization of bioactive film from pectin incorporated with gamma-aminobutyric acid. Int. J. Biol. Macromol. 2020, 147, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Bierhalz, A.C.K.; da Silva, M.A.; Kieckbusch, T.G. Natamycin release from alginate/pectin films for food packaging applications. J. Food Eng. 2012, 110, 18–25. [Google Scholar] [CrossRef]
- Lutz, R.; Aserin, A.; Wicker, L.; Garti, N. Structure and physical properties of pectins with block-wise distribution of carboxylic acid groups. Food Hydrocoll. 2009, 23, 786–794. [Google Scholar] [CrossRef]
- Kameda, T. Molecular structure of crude beeswax studied by solid-state 13C NMR. J. Insect Sci. 2004, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Cruces, F.; García, M.G.; Ochoa, N.A. Reduction of Water Vapor Permeability in Food Multilayer Biopackaging by Epitaxial Crystallization of Beeswax. Food Bioprocess Technol. 2021, 14, 1244–1255. [Google Scholar] [CrossRef]
- Eghbal, N.; Degraeve, P.; Oulahal, N.; Yarmand, M.S.; Mousavi, M.E.; Gharsallaoui, A. Low methoxyl pectin/sodium caseinate interactions and composite film formation at neutral pH. Food Hydrocoll. 2017, 69, 132–140. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B: Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of edible films and coatings with antimicrobial activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Li, Y. Structure–property modification of microcrystalline cellulose film using agar and propylene glycol alginate. J. Appl. Polym. Sci. 2017, 134, 45533. [Google Scholar] [CrossRef]
- Lorevice, M.V.; Otoni, C.G.; de Moura, M.R.; Mattoso, L.H.C. Chitosan nanoparticles on the improvement of thermal, barrier, and mechanical properties of high-and low-methyl pectin films. Food Hydrocoll. 2016, 52, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, R.S.; Mattoso, L.H.; De Moura, M.R. New edible bionanocomposite prepared by pectin and clove essential oil nanoemulsions. J. Nanosci. Nanotechnol. 2016, 16, 6540–6544. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H.J.I.j.o.b.m. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar]
- Hafila, K.; Jumaidin, R.; Ilyas, R.; Selamat, M.; Yusof, F.A.M. Effect of palm wax on the mechanical, thermal, and moisture absorption properties of thermoplastic cassava starch composites. Int. J. Biol. Macromol. 2022, 194, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ying, D.; Zhang, H.; Xu, X.; Chang, C. Self-Healable Hydrophobic Films Fabricated by Incorporating Natural Wax into Cellulose Matrix. Chem. Eng. J. 2022, 446, 136791. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Wongphan, P.; Promhuad, K.; Leelaphiwat, P.; Harnkarnsujarit, N. Morphology and permeability of bio-based poly (butylene adipate-co-terephthalate) (PBAT), poly (butylene succinate) (PBS) and linear low-density polyethylene (LLDPE) blend films control shelf-life of packaged bread. Food Control. 2022, 132, 108541. [Google Scholar] [CrossRef]
- Littlejohn, G.R.; Mansfield, J.C.; Parker, D.; Lind, R.; Perfect, S.; Seymour, M.; Smirnoff, N.; Love, J.; Moger, J. In vivo chemical and structural analysis of plant cuticular waxes using stimulated Raman scattering microscopy. Plant Physiol. 2015, 168, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Edwards, H.; Falk, M. Fourier-transform Raman spectroscopic study of unsaturated and saturated waxes. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 1997, 53, 2685–2694. [Google Scholar] [CrossRef]
- Farber, C.; Li, J.; Hager, E.; Chemelewski, R.; Mullet, J.; Rogachev, A.Y.; Kurouski, D. Complementarity of raman and infrared spectroscopy for structural characterization of plant epicuticular waxes. ACS Omega 2019, 4, 3700–3707. [Google Scholar] [CrossRef] [Green Version]
- Al-Amoudi, R.H.; Taylan, O.; Kutlu, G.; Can, A.M.; Sagdic, O.; Dertli, E.; Yilmaz, M.T. Characterization of chemical, molecular, thermal and rheological properties of medlar pectin extracted at optimum conditions as determined by Box-Behnken and ANFIS models. Food Chem. 2019, 271, 650–662. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Matějka, P.; Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Rösch, P.; Schmitt, M.; Popp, J.; Zdunek, A. Raman imaging of changes in the polysaccharides distribution in the cell wall during apple fruit development and senescence. Planta 2016, 243, 935–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighi, H.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Pulvirenti, A. Comparative analysis of blend and bilayer films based on chitosan and gelatin enriched with LAE (lauroyl arginate ethyl) with antimicrobial activity for food packaging applications. Food Packag. Shelf Life 2019, 19, 31–39. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. Imaging of polysaccharides in the tomato cell wall with Raman microspectroscopy. Plant. Methods 2014, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bichara, L.C.; Alvarez, P.E.; Bimbi, M.V.F.; Vaca, H.; Gervasi, C.; Brandán, S.A. Structural and spectroscopic study of a pectin isolated from citrus peel by using FTIR and FT-Raman spectra and DFT calculations. Infrared Phys. Technol. 2016, 76, 315–327. [Google Scholar] [CrossRef]





| Samples | Conductivity [μS/cm] | Surface Tension [mN·m−1] | Density [g·mL−1] | Viscosity [Pa·s] | Flow Index n |
|---|---|---|---|---|---|
| HMsol | 858.3 ± 28.3 b | 37.8 ± 0.5 a | 1.0164 ± 0.0007 a | 0.0578 ± 0.0001 a | 0.9834 ± 0.0004 c |
| LMsol | 1012.3 ± 42.0 d | 38.8 ± 0.3 ab | 1.0158 ± 0.0005 a | 0.2254 ± 0.0519 b | 0.7408 ± 0.0200 a |
| SFW-HMem | 940.7 ± 31.6 c | 43.6 ± 0.4 c | 1.0280 ± 0.0016 b | 0.0976 ± 0.0021 a | 0.9788 ± 0.0006 c |
| SFW-LMem | 64.9 ± 1.2 a | 39.9 ± 0.8 b | 1.0273 ± 0.0023 b | 0.2236 ± 0.0243 b | 0.8066 ± 0.0172 b |
| Film | Thickness (mm) | WVTR × 103 (g/h cm2) | WVP × 104 (g mm/KPa h cm2) |
|---|---|---|---|
| HM | 0.023 ± 0.003 ab | 9.54 ± 1.24 b | 0.51 ± 0.03 b |
| LM | 0.018 ± 0.003 a | 8.29 ± 0.46 b | 0.36 ± 0.04 a |
| SFW-HM | 0.031 ± 0.007 c | 6.60 ± 0.40 a | 0.41 ± 0.03 a |
| SFW-LM | 0.026 ± 0.006 bc | 6.43 ± 0.26 a | 0.40 ± 0.01 a |
| Film | Degree of Crystallinity (%) | Rq (nm) | Ra (nm) |
|---|---|---|---|
| HM | 8.6 ± 2.0 b | 10.8 ± 1.5 a | 7.6 ± 1.0 a |
| LM | 10.4 ± 2.6 b | 15.5 ± 2.1 b | 12.5 ± 1.7 b |
| SFW-HM | 1.7 ± 0.2 a | 59.0 ± 4.3 c | 43.9 ± 1.9 c |
| SFW-LM | 9.1 ± 0.4 b | 56.7 ± 4.5 c | 45.3 ± 4.2 c |
| Film | %E | TS (MPa) | Y (MPa) |
|---|---|---|---|
| HM | 6.12 ± 1.62 a | 9.08 ± 1.10 ab | 202.54 ± 8.56 c |
| LM | 5.07 ± 1.72 a | 6.84 ± 0.50 a | 171.98 ± 3.84 a |
| SFW-HM | 5.53 ± 1.25 a | 11.68 ± 2.20 b | 198.75 ± 1.35 bc |
| SFW-LM | 7.53 ± 0.34 a | 6.99 ± 0.56 a | 186.77 ± 6.13 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalapud, M.C.; Baümler, E.R.; Carelli, A.A.; Salgado-Cruz, M.d.l.P.; Morales-Sánchez, E.; Rentería-Ortega, M.; Calderón-Domínguez, G. Pectin Films with Recovered Sunflower Waxes Produced by Electrospraying. Membranes 2022, 12, 560. https://doi.org/10.3390/membranes12060560
Chalapud MC, Baümler ER, Carelli AA, Salgado-Cruz MdlP, Morales-Sánchez E, Rentería-Ortega M, Calderón-Domínguez G. Pectin Films with Recovered Sunflower Waxes Produced by Electrospraying. Membranes. 2022; 12(6):560. https://doi.org/10.3390/membranes12060560
Chicago/Turabian StyleChalapud, Mayra C., Erica R. Baümler, Amalia A. Carelli, Ma. de la Paz Salgado-Cruz, Eduardo Morales-Sánchez, Minerva Rentería-Ortega, and Georgina Calderón-Domínguez. 2022. "Pectin Films with Recovered Sunflower Waxes Produced by Electrospraying" Membranes 12, no. 6: 560. https://doi.org/10.3390/membranes12060560