A Membrane with Strong Resistance to Organic and Biological Fouling Using Graphene Oxide and D-Tyrosine as Modifiers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
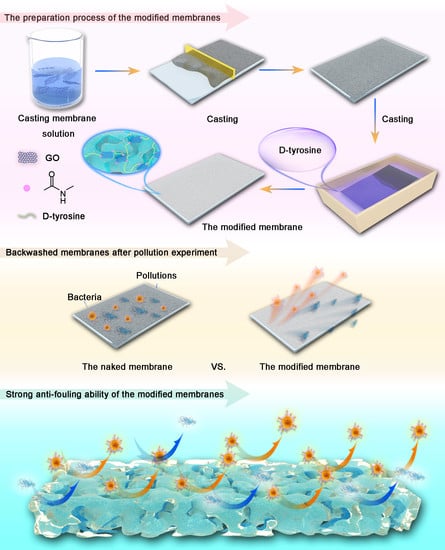
2.2. Preparation of PVDF, P-GO, P-GO-DAA Membranes
2.3. Characteristics of Membranes
2.4. The Permeability of the Membranes
2.5. Separate Performance and Antifouling Activity
2.6. Anti-Biofouling Activity
2.7. Characterization of Comprehensive Fouling and Biofouling Resistance Activity
3. Results and Discussion
3.1. Characterization of PVDF Membranes
3.2. Pure Water Permeability of the Membranes
3.3. Separate Performance of the Membranes
3.4. Antifouling Performance of the Membranes
3.5. Anti-Biofouling Performance of the Membranes
3.6. Antifouling and Anti-Biofouling Performance of the Membranes through Cycle Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Sun, M.; Zhao, Y.; Wang, C.; Ma, W.; Wong, M.S.; Elimelech, M. In Situ Electrochemical Generation of Reactive Chlorine Species for Efficient Ultrafiltration Membrane Self-Cleaning. Environ. Sci. Technol. 2020, 54, 6997–7007. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.Y.; Zhang, P.; Fang, L.F.; Zhu, B.K.; Wang, J.L.; Chen, J.H.; Abdallah, H. A positively charged tight UF membrane and its properties for removing trace metal cations via electrostatic repulsion mechanism. J. Hazard. Mater. 2019, 373, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Haghighat, N. Improvement of polyvinyl chloride nanofiltration membranes by incorporation of multiwalled carbon nanotubes modified with triethylenetetramine to use in treatment of dye wastewater. J. Environ. Manag. 2019, 242, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lin, Q.; Chen, S.; Chen, X. In-plane biaxial ratcheting behavior of PVDF UF membrane. Polym. Test. 2016, 50, 41–48. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Bai, J.; Li, J.; Wang, J.; Li, L.; Zhou, T.; Chen, S.; Rahim, M.; Zhou, B. Efficient denitrification and removal of natural organic matter, emerging pollutants simultaneously for RO concentrate based on photoelectrocatalytic radical reaction. Sep. Purif. Technol. 2020, 234, 116032. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Koutahzadeh, N.; Esfahani, A.R.; Firouzjaei, M.D.; Anderson, B.; Peck, L. A novel gold nanocomposite membrane with enhanced permeation, rejection and self-cleaning ability. J. Membr. Sci. 2019, 573, 309–319. [Google Scholar] [CrossRef]
- Liu, Q.; Li, L.; Pan, Z.; Dong, Q.; Xu, N.; Wang, T. Inorganic nanoparticles incorporated in polyacrylonitrile-based mixed matrix membranes for hydrophilic, ultrafast, and fouling-resistant ultrafiltration. J. Appl. Polym. Sci. 2019, 136, 47902. [Google Scholar] [CrossRef]
- Deng, H.; Zheng, Q.; Chen, H.; Huang, J.; Yan, H.; Ma, M.; Xia, M.; Pei, K.; Ni, H.; Ye, P. Graphene oxide/silica composite nanofiltration membrane: Adjustment of the channel of water permeation. Sep. Purif. Technol. 2022, 278, 119440. [Google Scholar] [CrossRef]
- Fan, G.; Chen, C.; Chen, X.; Li, Z.; Bao, S.; Luo, J.; Tang, D.; Yan, Z. Enhancing the antifouling and rejection properties of PVDF membrane by Ag3PO4-GO modification. Sci. Total Environ. 2021, 801, 149611. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Xie, Q.; Hong, Z.; Wu, C.; Yu, T.; Fang, H.; Xiong, Y.; Zhang, G.; Lu, Y.; Shao, W. Facile Strategy to Construct High-Performance Nanofiltration Membranes by Synergy of Graphene Oxide and Polyvinyl Alcohol. Ind. Eng. Chem. Res. 2020, 59, 19001–19011. [Google Scholar] [CrossRef]
- Rahimi, A.; Mahdavi, H. Zwitterionic-functionalized GO/PVDF nanocomposite membranes with improved anti-fouling properties. J. Water Process Eng. 2019, 32, 100960. [Google Scholar] [CrossRef]
- Wu, X.; Field, R.W.; Wu, J.J.; Zhang, K. Polyvinylpyrrolidone modified graphene oxide as a modifier for thin film composite forward osmosis membranes. J. Membr. Sci. 2017, 540, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Yuan, J.; Wu, H.; Cao, L.; Zhang, R.; Liu, Y.; Li, L.; Rahman, A.U.; Kasher, R.; Jiang, Z. Mixed Nanosheet Membranes Assembled from Chemically Grafted Graphene Oxide and Covalent Organic Frameworks for Ultra-high Water Flux. ACS Appl. Mater. Interfaces 2019, 11, 28978–28986. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Samal, A.K.; Altaee, A.; Deon, S.; Zhou, J.; Ghaffour, N. Preparation of fouling resistant and highly perm-selective novel PSf/GO-vanillin nanofiltration membrane for efficient water purification. J. Hazard. Mater. 2022, 421, 126744. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Cui, Z.; Li, J.; Zhang, L.; Mo, Y.; Dlamini, D.S.; Wang, H.; He, B.; Li, J.; Matsuyama, H. Ultra-low graphene oxide loading for water permeability, antifouling and antibacterial improvement of polyethersulfone/sulfonated polysulfone ultrafiltration membranes. J. Colloid. Interface Sci. 2019, 552, 319–331. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Li, J.; Li, J.; Bian, W.; Ji, S. Layer-by-layer self-assembly of polycation/GO nanofiltration membrane with enhanced stability and fouling resistance. Sep. Purif. Technol. 2016, 160, 123–131. [Google Scholar] [CrossRef]
- Khan, M.M.T.; Stewart, P.S.; Moll, D.J.; Mickols, W.E.; Burr, M.D.; Nelson, S.E.; Camper, A.K. Assessing biofouling on polyamide reverse osmosis (RO) membrane surfaces in a laboratory system. J. Membr. Sci. 2010, 349, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.-C.; Griebe, T.; Schaule, G.; Schmitt, J.; Tamachkiarowa, A. Biofouling—The Achille’s heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, M.; Wang, Z.; Hu, W.; Cao, J.; Wu, Z.-C. Uniqueness of biofouling in forward osmosis systems: Mechanisms and control. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1031–1066. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Sun, X.-F.; Qin, J.; Xia, P.-F.; Guo, B.-B.; Yang, C.-M.; Song, C.; Wang, S.-G. Graphene oxide–silver nanoparticle membrane for biofouling control and water purification. Chem. Eng. J. 2015, 281, 53–59. [Google Scholar] [CrossRef]
- Hu, J.Y.; Song, L.F.; Ong, S.L.; Phua, E.T.; Ng, W.J. Biofiltration pretreatment for reverse osmosis (RO) membrane in a water reclamation system. Chemosphere 2005, 59, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Asha, A.B.; Chen, Y.; Narain, R. Bioinspired dopamine and zwitterionic polymers for non-fouling surface engineering. Chem. Soc. Rev. 2021, 50, 11668–11683. [Google Scholar] [CrossRef]
- Guo, X.; Yang, H.; Liu, Q.; Liu, J.; Chen, R.; Zhang, H.; Yu, J.; Zhang, M.; Li, R.; Wang, J. A chitosan-graphene oxide/ZIF foam with anti-biofouling ability for uranium recovery from seawater. Chem. Eng. J. 2020, 382, 122850. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, Z.; Zhang, X.; Fan, S.; Gao, H.; Liu, C.; Zhou, Q.; Shao, H.; Wang, L.; Guo, X. Exploration on Optimized Control Way of D-Amino Acid for Efficiently Mitigating Membrane Biofouling of Membrane Bioreactor. Membranes 2021, 11, 612. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, S.; Xiao, K.; Huang, X. Enzymatic Cleaning Mitigates Polysaccharide-Induced Refouling of RO Membrane: Evidence from Foulant Layer Structure and Microbial Dynamics. Environ. Sci. Technol 2021, 55, 5453–5462. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Cao, S.; Chai, L.; Bottcher, T.; Kolter, R.; Clardy, J.; Losick, R. A self-produced trigger for biofilm disassembly that targets exopolysaccharide. Cell 2012, 149, 684–692. [Google Scholar] [CrossRef] [Green Version]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-amino acids trigger biofilm disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef] [Green Version]
- Xing, S.F.; Sun, X.F.; Taylor, A.A.; Walker, S.L.; Wang, Y.F.; Wang, S.G. D-amino acids inhibit initial bacterial adhesion: Thermodynamic evidence. Biotechnol. Bioeng. 2015, 112, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Wu, J.; Contreras, A.E.; Li, Q. Control of nanofiltration membrane biofouling by Pseudomonas aeruginosa using D-Tyrosine. J. Membr. Sci. 2012, 423–424, 487–494. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Zhang, N.; Wen, D.; Liu, C.; Li, Q. Inhibition of biofilm formation by D-Tyrosine: Effect of bacterial type and D-Tyrosine concentration. Water Res. 2016, 92, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Liu, Y. Biological control of microbial attachment: A promising alternative for mitigating membrane biofouling. Appl. Microbiol. Biotechnol. 2010, 86, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Y. D-Amino acid mitigated membrane biofouling and promoted biofilm detachment. J. Membr. Sci. 2011, 376, 266–274. [Google Scholar] [CrossRef]
- Yu, C.; Wu, J.; Zin, G.; Di Luccio, M.; Wen, D.; Li, Q. D-Tyrosine loaded nanocomposite membranes for environmental-friendly, long-term biofouling control. Water Res. 2018, 130, 105–114. [Google Scholar] [CrossRef]
- Lam, H.; Oh, D.C.; Cava, F.; Takacs, C.N.; Clardy, J.; de Pedro, M.A.; Waldor, M.K. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science 2009, 325, 1552–1555. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Fan, S.; Hu, Y.; Fu, X.; Shao, H.; Zhou, Q. A novel membrane biofouling mitigation strategy of D-amino acid supported by polydopamine and halloysite nanotube. J. Membr. Sci. 2019, 579, 131–140. [Google Scholar] [CrossRef]
- Khan, R.; Khan, M.K.; Wang, H.; Xiao, K.; Huang, X. Grafting d-amino acid onto MF polyamide nylon membrane for biofouling control using biopolymer alginate dialdehyde as a versatile platform. Sep. Purif. Technol. 2020, 231, 115891. [Google Scholar] [CrossRef]
- Jiang, B.-B.; Sun, X.-F.; Wang, L.; Wang, S.-Y.; Liu, R.-D.; Wang, S.-G. Polyethersulfone membranes modified with D-Tyrosine for biofouling mitigation: Synergistic effect of surface hydrophility and anti-microbial properties. Chem. Eng. J. 2017, 311, 135–142. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Gad-Allah, T.A.; El-Kalliny, A.S.; Ahmed, S.I.A.; Souaya, E.R.; Badawy, M.I.; Ulbricht, M. Fabrication of modified polyethersulfone membranes for wastewater treatment by submerged membrane bioreactor. Sep. Purif. Technol. 2017, 175, 36–46. [Google Scholar] [CrossRef]
- Miller, D.J.; Araujo, P.A.; Correia, P.B.; Ramsey, M.M.; Kruithof, J.C.; van Loosdrecht, M.C.; Freeman, B.D.; Paul, D.R.; Whiteley, M.; Vrouwenvelder, J.S. Short-term adhesion and long-term biofouling testing of polydopamine and poly(ethylene glycol) surface modifications of membranes and feed spacers for biofouling control. Water Res. 2012, 46, 3737–3753. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Han, Y.; Tai, S. Biodiesel production using biguanide-functionalized hydroxyapatite-encapsulated-γ-Fe2O3 nanoparticles. Fuel 2017, 210, 83–90. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Altaee, A.; Déon, S.; Zhou, J. Preparation of novel high permeability and antifouling polysulfone-vanillin membrane. Desalination 2020, 496, 114759. [Google Scholar] [CrossRef]
- Khalili, D. Graphene oxide: A promising carbocatalyst for the regioselective thiocyanation of aromatic amines, phenols, anisols and enolizable ketones by hydrogen peroxide/KSCN in water. New J. Chem. 2016, 40, 2547–2553. [Google Scholar] [CrossRef]
- Sri Abirami Saraswathi, M.S.; Rana, D.; Divya, K.; Gowrishankar, S.; Nagendran, A. Versatility of hydrophilic and antifouling PVDF ultrafiltration membranes tailored with polyhexanide coated copper oxide nanoparticles. Polym. Test. 2020, 84, 106367. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.-S. Molecular design of the morphology and pore size of PVDF hollow fiber membranes for ethanol–water separation employing the modified pore-flow concept. J. Membr. Sci. 2011, 374, 67–82. [Google Scholar] [CrossRef]
- Al-Jeshi, S.; Neville, A. An investigation into the relationship between flux and roughness on RO membranes using scanning probe microscopy. Desalination 2006, 189, 221–228. [Google Scholar] [CrossRef]
- Feng, S.; Yu, G.; Cai, X.; Eulade, M.; Lin, H.; Chen, J.; Liu, Y.; Liao, B.Q. Effects of fractal roughness of membrane surfaces on interfacial interactions associated with membrane fouling in a membrane bioreactor. Bioresour. Technol. 2017, 244, 560–568. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Effect of TiO2 addition on the fabrication of ceramic membrane supports: A study on the separation of oil droplets and bovine serum albumin (BSA) from its solution. Desalination 2011, 279, 104–114. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, R.; Wang, S.; Gao, S.; Tian, J. Membrane fouling behaviors of ceramic hollow fiber microfiltration (MF) membranes by typical organic matters. Sep. Purif. Technol. 2021, 274, 118951. [Google Scholar] [CrossRef]
- Tian, J.-Y.; Ernst, M.; Cui, F.; Jekel, M. Effect of different cations on UF membrane fouling by NOM fractions. Chem. Eng. J. 2013, 223, 547–555. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, R.; Liu, Y.; He, M.; Su, Y.; Gao, C.; Jiang, Z. Antifouling membrane surface construction: Chemistry plays a critical role. J. Membr. Sci. 2018, 551, 145–171. [Google Scholar] [CrossRef]
- Khalid, A.; Abdel-Karim, A.; Ali Atieh, M.; Javed, S.; McKay, G. PEG-CNTs nanocomposite PSU membranes for wastewater treatment by membrane bioreactor. Sep. Purif. Technol. 2018, 190, 165–176. [Google Scholar] [CrossRef]














| Name of the Solution | Contents and Concentrations |
|---|---|
| BSA (Bull Serum Albumin) | BSA, 100 mg/L |
| SA (Sodium alga Acid) | SA, 50 mg/L |
| HA (Humic Acid) | HA, 50 mg/L |
| Bi (Binary pollutants) | BSA, 100 mg/L SA, 50 mg/L |
| Tri (Triple pollutants) | BSA, 100 mg/L SA, 50 mg/L HA, 50 mg/L |
| AS (Actual Sewage) | Effluent from a secondary sedimentation tank in a sewage plant in Beijing, TOC: 182 mg/L |
| Synthetic pollutants solution | BSA, 100 mg/L SA, 100 mg/L HA, 50 mg/L E. coli, 3.78 × 107 CFU |
| Membrane | Average Pore Size (nm) | Porosity (%) |
|---|---|---|
| PVDF | 16.9 (±2.1) | 74.5 (±1.4) |
| P-GO | 16.2 (±1.6) | 79.1 (±1.8) |
| P-GO-50 | 15.8 (±1.3) | 80.3 (±2.5) |
| P-GO-100 | 15.7 (±1.8) | 81.0 (±2.3) |
| P-GO-150 | 14.4 (±2.0) | 79.6 (±2.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Zhang, Y.; Chen, F.; Chai, Y. A Membrane with Strong Resistance to Organic and Biological Fouling Using Graphene Oxide and D-Tyrosine as Modifiers. Membranes 2022, 12, 486. https://doi.org/10.3390/membranes12050486
Guo J, Zhang Y, Chen F, Chai Y. A Membrane with Strong Resistance to Organic and Biological Fouling Using Graphene Oxide and D-Tyrosine as Modifiers. Membranes. 2022; 12(5):486. https://doi.org/10.3390/membranes12050486
Chicago/Turabian StyleGuo, Jiarui, Yan Zhang, Fenghua Chen, and Yuman Chai. 2022. "A Membrane with Strong Resistance to Organic and Biological Fouling Using Graphene Oxide and D-Tyrosine as Modifiers" Membranes 12, no. 5: 486. https://doi.org/10.3390/membranes12050486