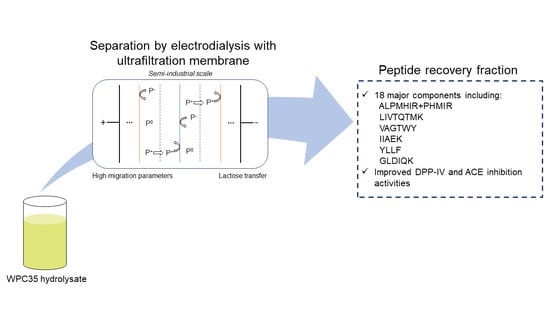
Semi-Industrial Production of a DPP-IV and ACE Inhibitory Peptide Fraction from Whey Protein Concentrate Hydrolysate by Electrodialysis with Ultrafiltration Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Whey Protein Hydrolysate
2.2. Isolation of Peptide from the Whey Protein Hydrolysate
2.2.1. Electrodialysis Cell
2.2.2. Protocol
2.3. Demineralization of Initial WPH, Final WPH and Peptide Recovery Fraction
2.3.1. Electrodialysis Cell
2.3.2. Protocol
2.4. Analyses
2.4.1. Conductivity and Local Electric Field Strength
2.4.2. Peptide Recovery, Yield and Migration
2.4.3. Individual Peptide Migration
2.4.4. Total Peptide Content
2.4.5. Peptide Relative Abundance
2.4.6. Bioactivity Assay
- DPP-IV inhibitory activity
- b.
- ACE inhibitory activity
- c.
- Antioxidant activity
2.4.7. Lactose Content
2.5. Statistical Analysis
3. Results and Discussion
3.1. Process Parameters
3.1.1. Conductivity of the Solutions
3.1.2. Local Electric Field Strength
3.2. Peptide Recovery, Yield and Migration Rate
3.3. Peptide Identification and Semi-Quantification
3.4. Bioactivities
3.5. Lactose Transfer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dullius, A.; Goettert, M.I.; de Souza, C.F.V. Whey Protein Hydrolysates as a Source of Bioactive Peptides for Functional Foods—Biotechnological Facilitation of Industrial Scale-Up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- Pouliot, Y.; Wijers, M.C.; Gauthier, S.F.; Nadeau, L. Fractionation of Whey Protein Hydrolysates Using Charged UF/NF Membranes. J. Membr. Sci. 1999, 158, 105–114. [Google Scholar] [CrossRef]
- Hannu, K.; Pihlanto, A. Bioactive Peptides: Production and Functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Dlask, O.; Vaclavikova, N. Electrodialysis with Ultrafiltration Membranes for Peptide Separation. Chem. Pap. 2018, 72, 261–271. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Q.; Lu, H.; Wang, J.; Zhao, J.; Li, P. Electrodialysis with Porous Membrane for Bioproduct Separation: Technology, Features, and Progress. Food Res. Int. 2020, 137, 109343. [Google Scholar] [CrossRef]
- Bazinet, L.; Geoffroy, T.R. Electrodialytic Processes: Market Overview, Membrane Phenomena, Recent Developments and Sustainable Strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef]
- Durand, R.; Fraboulet, E.; Marette, A.; Bazinet, L. Separation and Purification Technology Simultaneous Double Cationic and Anionic Molecule Separation from Herring Milt Hydrolysate and Impact on Resulting Fraction Bioactivities. Sep. Purif. Technol. 2019, 210, 431–441. [Google Scholar] [CrossRef]
- Przybylski, R.; Bazinet, L.; Firdaous, L.; Kouach, M.; Goossens, J.-F.; Dhulster, P.; Nedjar, N. Harnessing Slaughterhouse By-Products: From Wastes to High-Added Value Natural Food Preservative. Food Chem. 2020, 304, 125448. [Google Scholar] [CrossRef]
- He, R.; Girgih, A.T.; Rozoy, E.; Bazinet, L.; Ju, X.; Aluko, R.E. Selective Separation and Concentration of Antihypertensive Peptides from Rapeseed Protein Hydrolysate by Electrodialysis with Ultrafiltration Membranes. Food Chem. 2016, 197, 1008–1014. [Google Scholar] [CrossRef]
- Kadel, S.; Daigle, G.; Thibodeau, J.; Perreault, V.; Pellerin, G.; Lainé, C.; Bazinet, L. How Physicochemical Properties of Filtration Membranes Impact Peptide Migration and Selectivity during Electrodialysis with Filtration Membranes: Development of Predictive Statistical Models and Understanding of Mechanisms Involved. J. Membr. Sci. 2021, 619. [Google Scholar] [CrossRef]
- Suwal, S.; Roblet, C.; Doyen, A.; Amiot, J.; Beaulieu, L.; Legault, J.; Bazinet, L. Electrodialytic Separation of Peptides from Snow Crab By-Product Hydrolysate: Effect of Cell Configuration on Peptide Selectivity and Local Electric Field. Sep. Purif. Technol. 2014, 127, 29–38. [Google Scholar] [CrossRef]
- Poulin, J.-F.; Amiot, J.; Bazinet, L. Impact of Feed Solution Flow Rate on Peptide Fractionation by Electrodialysis with Ultrafiltration Membrane. J. Agric. Food Chem. 2008, 56, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Roblet, C.; Doyen, A.; Amiot, J.; Bazinet, L. Impact of PH on Ultrafiltration Membrane Selectivity during Electrodialysis with Ultrafiltration Membrane (EDUF) Purification of Soy Peptides from a Complex Matrix. J. Membr. Sci. 2013, 435, 207–217. [Google Scholar] [CrossRef]
- Geoffroy, T.R.; Bernier, M.E.; Thibodeau, J.; Francezon, N.; Beaulieu, L.; Mikhaylin, S.; Langevin, M.E.; Lutin, F.; Bazinet, L. Semi-Industrial Scale-up of EDUF Technology for the Electroseparation of Bioactive Cationic Peptides: Impact of Process Parameters and Cell. J. Membr. Sci. 2022, 641, 119856. [Google Scholar] [CrossRef]
- Geoffroy, T.R.; Thibodeau, J.; Faucher, M.; Langevin, M.È.; Lutin, F.; Bazinet, L. Relationship between Feed Concentration and Biactive Cationic Peptide Recovery: Impact on Ecoefficiency of EDUF at Semi-Industrial Scale. Sep. Purif. Technol. 2022, 286, 120403. [Google Scholar] [CrossRef]
- Kadel, S.; Pellerin, G.; Thibodeau, J.; Perreault, V.; Lainé, C.; Bazinet, L. How Molecular Weight Cut-Offs and Physicochemical Properties of Polyether Sulfone Membranes Affect Peptide Migration and Selectivity during Electrodialysis with Filtration Membranes. Membranes 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Galier, S.; Balmann, H.R. Study of Biomolecules Separation in an Electrophoretic Membrane Contactor. J. Membr. Sci. 2004, 241, 79–87. [Google Scholar] [CrossRef]
- Maubois, J.-L.; Lorient, D. Dairy Proteins and Soy Proteins in Infant Foods Nitrogen-to-Protein Conversion Factors. Dairy Sci. Technol. 2016, 96, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Silveira, S.T.; Martínez-maqueda, D.; Recio, I.; Hernández-ledesma, B. Dipeptidyl Peptidase-IV Inhibitory Peptides Generated by Tryptic Hydrolysis of a Whey Protein Concentrate Rich in b-Lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [CrossRef]
- Hayakari, M.; Kondo, Y.; Izumi, H. And Simple Spectrophotometric Assay of Enzyme. Anal. Biochem. 1978, 84, 361–369. [Google Scholar] [CrossRef]
- Hell, A.; Labrie, S.; Beaulieu, L. Effect of Seaweed Flakes Addition on the Development of Bioactivities in Functional Camembert-Type Cheese. Int. J. Food Sci. Technol. 2018, 53, 1054–1064. [Google Scholar] [CrossRef] [Green Version]
- Held, P. Performing Oxygen Radical Absorbance Capacity Assays with SynergyTM HT; Application Note; BioTek Instruments, Inc.: Winooksi, VT, USA, 2006. [Google Scholar]
- ISO 22662; Milk and Milk Products-Determining of Lactose Content by High-Performance Liquid Chromatography (Reference Method). Organization of International Standardization: Geneva, Switzerland, 2007.
- Persico, M.; Dhulster, P.; Bazinet, L. Redundancy Analysis for Determination of the Main Physicochemical Characteristics of Filtration Membranes Explaining Their Fouling by Peptides. J. Membr. Sci. 2018, 563, 708–717. [Google Scholar] [CrossRef]
- Persico, M.; Daigle, G.; Kadel, S.; Perreault, V.; Pellerin, G. Predictive Models for Determination of Peptide Fouling Based on the Physicochemical Characteristics of Filtration Membranes. Sep. Purif. Technol. 2020, 240, 116602. [Google Scholar] [CrossRef]
- Power, O.; Fernández, A.; Norris, R.; Riera, F.A.; Fitzgerald, R.J. Selective Enrichment of Bioactive Properties during Ultrafiltration of a Tryptic Digest of β-Lactoglobulin. J. Funct. Foods 2014, 9, 38–47. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Mazzocchi, C.; Paolella, S.; Fitzgerald, R.J. Release of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides from Milk Protein Isolate (MPI) during Enzymatic Hydrolysis. Food Res. Int. 2017, 94, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Engel, M.; Hoffmann, T.; Wagner, L.; Wermann, M.; Heiser, U.; Kiefersauer, R.; Huber, R.; Bode, W.; Demuth, H.; Brandstetter, H. The Crystal Structure of Dipeptidyl Peptidase IV (CD26) Reveals Its Functional Regulation and Enzymatic Mechanism. Proc. Natl. Acad. Sci. USA 2003, 100, 5063–5068. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; Fitzgerald, R.J. Susceptibility of Milk Protein-Derived Peptides to Dipeptidyl Peptidase IV (DPP-IV) Hydrolysis. Food Chem. 2014, 145, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Tonouchi, H.; Takahashi, R.R.; Kitazawa, H.; Kawai, Y.; Negishi, H.; Saito, T. Structural Analysis of a New Antihypertensive Peptide (β-Lactosin B) Isolated from a Commercial Whey Product. J. Dairy Sci. 2004, 87, 1967–1974. [Google Scholar] [CrossRef] [Green Version]
- Pihlanto-Leppälä, A.; Rokka, T.; Korhonen, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Bovine Milk Proteins. Int. Dairy J. 1998, 8, 325–331. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A.; Koskinen, P.; Piilola, K.; Tupasela, T.; Korhonen, H. Angiotensin I-Converting Enzyme Inhibitory Properties of Whey Protein Digests: Concentration and Characterization of Active Peptides. J. Dairy Res. 2000, 67, 53–64. [Google Scholar] [CrossRef]
- Mullaly, M.M.; Meisel, H.; Fitzgerald, R.J. Synthetic Peptides Corresponding to Alpha-Lactalbumin and Beta-Lactoglobulin Sequences with Angiotensin-I-Converting Enzyme Inhibitory Activity. Biol. Chem. Hoppe. Seyler. 1996, 337, 259–260. [Google Scholar]
- De Boer, R. Information Sheets. In From Milk By-Products to Milk Ingredients: Upgrading the Cycle; John Wiley and Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- De Boer, R. Vital Membrane Processes. In From Milk By-Products to Milk Ingredients: Upgrading the Cycle; John Wiley and Sons: Hoboken, NJ, USA, 2014. [Google Scholar]





| Peptide Sequence | Observed Mass (Da) | Retention Time (min) | Net Charge at pH7 | Peptide Source | Relative Abundance in Peptide Recovery Fraction * | Relative Abundance in Initial WPH ** | Concentration factor in the Peptide Recovery Fraction | Final Migration Rate |
|---|---|---|---|---|---|---|---|---|
| VY | 280.14 | 2.5 | 0 | b-lg (41–42) | 3.75 ± 1.25 | 2.43 ± 0.48 | 1.53 ± 0.37 | 22.78 ± 2.24 |
| ILDK + AEK+IIAEK | 487.30, 346.15, 572.35 | 2.8 | 0, 0, 0 | a-la (95–98), b-lg (73–75), b-lg (71–75) | 4.95 ± 0.41 | 4.52 ± 0.80 | 1.12 ± 0.24 | 81.55 ± 2.47 |
| DAQSAPLR | 856.44 | 4.04 | 0 | b-lg (33–40) | 3.53 ± 0.26 | 3.43 ± 0.19 | 1.03 ± 0.09 | 52.13 ± 10.05 |
| IVTQTMK | 819.45 | 4.4 | 1 | b-lg (2–8) | 1.41 ± 0.11 | 1.14 ± 0.06 | 1.25 ± 0.14 | 27.60 ± 5.98 |
| IDALNENK | 915.47 | 5.1 | −1 | b-lg (84–91) | 0.45 ± 0.00 | 1.43 ± 0.03 | 0.31 ± 0.01 | 72.32 ± 16.94 |
| GLDIQK | 672.38 | 5.6 | 0 | b-lg (9–14) | 3.08 ± 0.06 | 3.50 ± 0.04 | 0.88 ± 0.01 | 37.06 ± 8.31 |
| ALPMHIR + PMHIR | 836.47, 656.35 | 7.91 | 1.1, 1.1 | b-lg (142–148), b-lg (144–148) | 1.66 ± 0.14 | 0.22 ± 0.00 | 7.52 ± 0.63 | 24.34 ± 1.84 |
| LIVTQTMK | 932.54 | 8.26 | 1 | b-lg (1–8) | 5.55 ± 0.28 | 3.88 ± 0.11 | 1.43 ± 0.03 | 8.86 ± 2.84 |
| ALPM + VGINY | 430.22, 564.29 | 8.63 | 0, 0 | b-lg (142–145), a-la (99–103) | 5.41 ± 0.08 | 3.33 ± 0.42 | 1.64 ± 0.24 | 3.84 ± 0.64 |
| VLVLDTDYKK | 1192.67 | 9.32 | 0 | b-lg (92–101) | 0.80 ± 0.05 | 3.14 ± 0.53 | 0.23 ± 0.03 | 14.68 ± 0.88 |
| VAGTWY | 695.33 | 10.62 | 0 | b-lg (15–20) | 28.04 ± 0.65 | 13.26 ± 0.35 | 2.11 ± 0.01 | 18.69 ± 1.19 |
| VLVLDTDYK | 1064.57 | 11.25 | -1 | b-lg (92–100) | 1.02 ± 0.17 | 2.55 ± 0.31 | 0.40 ± 0.05 | 3.20 ± 0.52 |
| TKIPAVF | 774.46 | 12.27 | 1 | b-lg (76–82) | 5.94 ± 0.36 | 2.61 ± 0.23 | 2.28 ± 0.08 | 66.01 ± 4.76 |
| IC50 DPP-IV Inhibition | IC50 ACE Inhibition | ORAC | |
|---|---|---|---|
| Demineralized initial WPH | 0.9734 ± 0.0463 a | 44.11 ± 0.43 a | 716.2 ± 96.8 a |
| Demineralized final WPH | 1.2175 ± 0.3781 a | 22.96 ± 0.58 b | 804.2 ± 234.3 a |
| Demineralized peptide recovery fraction | 0.5029 ± 0.0796 b | 10.62 ± 1.13 c | 824.8 ± 80.6 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faucher, M.; Geoffroy, T.R.; Thibodeau, J.; Gaaloul, S.; Bazinet, L. Semi-Industrial Production of a DPP-IV and ACE Inhibitory Peptide Fraction from Whey Protein Concentrate Hydrolysate by Electrodialysis with Ultrafiltration Membrane. Membranes 2022, 12, 409. https://doi.org/10.3390/membranes12040409
Faucher M, Geoffroy TR, Thibodeau J, Gaaloul S, Bazinet L. Semi-Industrial Production of a DPP-IV and ACE Inhibitory Peptide Fraction from Whey Protein Concentrate Hydrolysate by Electrodialysis with Ultrafiltration Membrane. Membranes. 2022; 12(4):409. https://doi.org/10.3390/membranes12040409
Chicago/Turabian StyleFaucher, Mélanie, Thibaud R. Geoffroy, Jacinthe Thibodeau, Sami Gaaloul, and Laurent Bazinet. 2022. "Semi-Industrial Production of a DPP-IV and ACE Inhibitory Peptide Fraction from Whey Protein Concentrate Hydrolysate by Electrodialysis with Ultrafiltration Membrane" Membranes 12, no. 4: 409. https://doi.org/10.3390/membranes12040409