Chronopotentiometric Evaluation of Ionization Degree and Dissociation Constant of Imidazolium-Based Ionic Liquid [C6Meim][NTf2] in Polymeric Plasticized Membranes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. NMR Studies of Pure IL [C6Meim][NTf2]
3.2. Mechanical Properties of the IL-Doped PVC Membranes
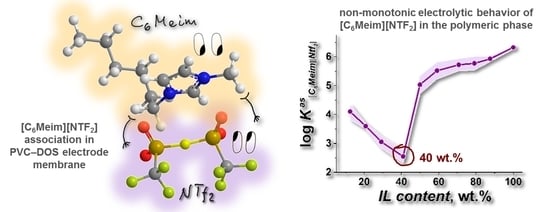
3.3. Electrochemical and NMR Studies of IL-Doped Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, R.; Bresser, D.; Saraf, M.; Gerlach, P.; Balducci, A.; Kunz, S.; Schröder, D.; Passerini, S.; Chen, J. A Comparative Review of Electrolytes for Organic-Material-Based Energy-Storage Devices Employing Solid Electrodes and Redox Fluids. ChemSusChem 2020, 13, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, M.A.; Mohebbi, A.; Pazhohan, A.; Nemati, M.; Afshar Mogaddam, M.R. Air–assisted liquid–liquid microextraction; principles and applications with analytical instruments. TrAC Trends Anal. Chem. 2020, 122, 115734. [Google Scholar] [CrossRef]
- Treder, N.; Bączek, T.; Wychodnik, K.; Rogowska, J.; Wolska, L.; Plenis, A. The Influence of Ionic Liquids on the Effectiveness of Analytical Methods Used in the Monitoring of Human and Veterinary Pharmaceuticals in Biological and Environmental Samples—Trends and Perspectives. Molecules 2020, 25, 286. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, A.; Byrne, R.; Diamond, D.; Fraser, K.J. Stimuli Responsive Ionogels for Sensing Applications—An Overview. Membranes 2012, 2, 16–39. [Google Scholar] [CrossRef] [Green Version]
- Behera, K.; Pandey, S.; Kadyan, A.; Pandey, S. Ionic liquid-based optical and electrochemical carbon dioxide sensors. Sensors 2015, 15, 30487–30503. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.; Zeng, X. Interfacial composition, structure, and properties of ionic liquids and conductive polymers for the construction of chemical sensors and biosensors: A perspective. Curr. Opin. Electrochem. 2020, 23, 47–56. [Google Scholar] [CrossRef]
- Galiullin, T.M.; Pokhvishcheva, N.V.; Kalinichev, A.V.; Peshkova, M.A. Evaluation of Ionic Liquids Based on Amino Acid Anions for Use in Liquid-junction Free Reference Electrodes. Electroanalysis 2019, 31, 1708–1718. [Google Scholar] [CrossRef]
- Wardak, C.; Grabarczyk, M. Single-piece all-solid-state Co(II) ion-selective electrode for cobalt monitoring in real samples. Int. Agrophys. 2019, 1, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Oter, O.; Ertekin, K.; Topkaya, D.; Alp, S. Room temperature ionic liquids as optical sensor matrix materials for gaseous and dissolved CO2. Sens. Actuators B Chem. 2006, 117, 295–301. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Aguayo-López, M.L.; Vargas-Sansalvador, I.d.P.; Capitán-Vallvey, L.F. Ionic liquids on optical sensors for gaseous carbon dioxide. Anal. Bioanal. Chem. 2018, 410, 5931–5939. [Google Scholar] [CrossRef]
- Absalan, G.; Asadi, M.; Kamran, S.; Torabi, S.; Sheikhian, L. Design of a cyanide ion optode based on immobilization of a new Co(III) Schiff base complex on triacetylcellulose membrane using room temperature ionic liquids as modifiers. Sens. Actuators B Chem. 2010, 147, 31–36. [Google Scholar] [CrossRef]
- Zhang, T.; Lai, C.-Z.; Fierke, M.A.; Stein, A.; Bühlmann, P. Advantages and Limitations of Reference Electrodes with an Ionic Liquid Junction and Three-Dimensionally Ordered Macroporous Carbon as Solid Contact. Anal. Chem. 2012, 84, 7771–7778. [Google Scholar] [CrossRef] [PubMed]
- Kokorin, A. (Ed.) Ionic Liquids: Applications and Perspectives; InTech: London, UK, 2011; ISBN 978-953-307-248-7. [Google Scholar]
- Cicmil, D.; Anastasova, S.; Kavanagh, A.; Diamond, D.; Mattinen, U.; Bobacka, J.; Lewenstam, A.; Radu, A. Ionic Liquid-Based, Liquid-Junction-Free Reference Electrode. Electroanalysis 2011, 23, 1881–1890. [Google Scholar] [CrossRef]
- Shvedene, N.V.; Chernyshov, D.V.; Pletnev, I.V. Ionic liquids in electrochemical sensors. Russ. Chem. J. 2008, LII, 80–91. [Google Scholar] [CrossRef]
- Shvedene, N.V.; Chernyshov, D.V.; Khrenova, M.G.; Formanovsky, A.A.; Baulin, V.E.; Pletnev, I.V. Ionic Liquids Plasticize and Bring Ion-Sensing Ability to Polymer Membranes of Selective Electrodes. Electroanalysis 2006, 18, 1416–1421. [Google Scholar] [CrossRef]
- Muginova, S.V.; Myasnikova, D.A.; Kazarian, S.G.; Shekhovtsova, T.N. Applications of ionic liquids for the development of optical chemical sensors and biosensors. Anal. Sci. 2017, 33, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Zuliani, C.; Matzeu, G.; Diamond, D. A potentiometric disposable sensor strip for measuring pH in saliva. Electrochim. Acta 2014, 132, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Shibata, M.; Sakaida, H.; Kakiuchi, T. Determination of the activity of hydrogen ions in dilute sulfuric acids by use of an ionic liquid salt bridge sandwiched by two hydrogen electrodes. Anal. Chem. 2011, 83, 164–168. [Google Scholar] [CrossRef]
- Vincze, A.; Horvai, G. The design of reference electrodes without liquid junction. Electrochem. Soc. Proc. 1997, 97–19, 550–555. [Google Scholar]
- Lindner, E.; Guzinski, M.; Khan, T.A.; Pendley, B.D. Reference Electrodes with Ionic Liquid Salt Bridge: When Will These Innovative Novel Reference Electrodes Gain Broad Acceptance? ACS Sens. 2019, 4, 549–561. [Google Scholar] [CrossRef]
- Pokhvishcheva, N.V.; Peshkova, M.A. Ionic Liquids as Plasticizers for Optodes. Mosc. Univ. Chem. Bull. 2020, 75, 115–120. [Google Scholar] [CrossRef]
- Anthony, J.L.; Maginn, E.J.; Brennecke, J.F. Solubilities and Thermodynamic Properties of Gases in the Ionic Liquid 1-n-Butyl-3-methylimidazolium Hexafluorophosphate. J. Phys. Chem. B 2002, 106, 7315–7320. [Google Scholar] [CrossRef]
- Noda, A.; Susan, M.A.B.H.; Kudo, K.; Mitsushima, S.; Hayamizu, K.; Watanabe, M. Brønsted acid-base ionic liquids as proton-conducting nonaqueous electrolytes. J. Phys. Chem. B 2003, 107, 4024–4033. [Google Scholar] [CrossRef]
- Bešter-Rogač, M.; Fedotova, M.V.; Kruchinin, S.E.; Klähn, M. Mobility and association of ions in aqueous solutions: The case of imidazolium based ionic liquids. Phys. Chem. Chem. Phys. 2016, 18, 28594–28605. [Google Scholar] [CrossRef] [Green Version]
- Umecky, T.; Saito, Y.; Matsumoto, H. Ion Mobility of 1-Ethyl-3-methylimidazolium Tetrafluoroborate and 1-Ethyl-3-methylimidazolium Bis(trifluorosulfonyl)amide Ionic Liquids. ECS Trans. 2019, 25, 23–29. [Google Scholar] [CrossRef]
- Tokuda, H.; Tsuzuki, S.; Susan, M.A.B.H.; Hayamizu, K.; Watanabe, M. How Ionic Are Room-Temperature Ionic Liquids? An Indicator of the Physicochemical Properties. J. Phys. Chem. B 2006, 110, 19593–19600. [Google Scholar] [CrossRef]
- McDaniel, J.G.; Son, C.Y. Ion Correlation and Collective Dynamics in BMIM/BF 4 -Based Organic Electrolytes: From Dilute Solutions to the Ionic Liquid Limit. J. Phys. Chem. B 2018, 122, 7154–7169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 1. General Characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef]
- Marciniak, A. The Solubility Parameters of Ionic Liquids. Int. J. Mol. Sci. 2010, 11, 1973–1990. [Google Scholar] [CrossRef] [Green Version]
- Kakiuchi, T. Mutual Solubility of Hydrophobic Ionic Liquids and Water in Liquid-Liquid Two-phase Systems for Analytical Chemistry. Anal. Sci. 2008, 24, 1221–1230. [Google Scholar] [CrossRef] [Green Version]
- Price, W.S. Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion: Part II. Experimental aspects. Concepts Magn. Reson. 1998, 10, 197–237. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Ye, Q.; Keresztes, Z.; Horvai, G. Characterization of the Outmost Surface of Ion-Selective Solvent Polymeric PVC Membranes and Protein Adsorption. Electroanalysis 1999, 11, 729–734. [Google Scholar] [CrossRef]
- Mikhelson, K.N.; Lutov, V.M.; Sulko, K.; Stefanova, O.K. Single-pulse galvanostatic investigation of valinomycin containing membranes. Sov. Electrochem. 1988, 24, 1369. [Google Scholar]
- Peshkova, M.A.; Korobeynikov, A.I.; Mikhelson, K.N. Estimation of ion-site association constants in ion-selective electrode membranes by modified segmented sandwich membrane method. Electrochim. Acta 2008, 53, 5819–5826. [Google Scholar] [CrossRef]
- Berzins, T.; Delahay, P. Oscillographic Polarographic Waves for the Reversible Deposition of Metals on Solid Electrodes. J. Am. Chem. Soc. 1953, 75, 555–559. [Google Scholar] [CrossRef]
- Nägele, M.; Pretsch, E. New method for determining the concentration of ionic impurities in solvent polymeric membranes. Mikrochim. Acta 1995, 121, 269–279. [Google Scholar] [CrossRef]
- Qin, Y.; Bakker, E. Quantification of the Concentration of Ionic Impurities in Polymeric Sensing Membranes with the Segmented Sandwich Technique. Anal. Chem. 2001, 73, 4262–4267. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokhvishcheva, N.V.; Gigiadze, E.K.; Kalinichev, A.V.; Ievlev, A.V.; Tyutyukin, K.V.; Peshkova, M.A. Chronopotentiometric Evaluation of Ionization Degree and Dissociation Constant of Imidazolium-Based Ionic Liquid [C6Meim][NTf2] in Polymeric Plasticized Membranes. Membranes 2022, 12, 130. https://doi.org/10.3390/membranes12020130
Pokhvishcheva NV, Gigiadze EK, Kalinichev AV, Ievlev AV, Tyutyukin KV, Peshkova MA. Chronopotentiometric Evaluation of Ionization Degree and Dissociation Constant of Imidazolium-Based Ionic Liquid [C6Meim][NTf2] in Polymeric Plasticized Membranes. Membranes. 2022; 12(2):130. https://doi.org/10.3390/membranes12020130
Chicago/Turabian StylePokhvishcheva, Nadezhda V., Elizaveta K. Gigiadze, Andrey V. Kalinichev, Alexandr V. Ievlev, Konstantin V. Tyutyukin, and Maria A. Peshkova. 2022. "Chronopotentiometric Evaluation of Ionization Degree and Dissociation Constant of Imidazolium-Based Ionic Liquid [C6Meim][NTf2] in Polymeric Plasticized Membranes" Membranes 12, no. 2: 130. https://doi.org/10.3390/membranes12020130