3.1. Chemical Components of EOs
Chemical components and their percentages of the total EO content in six EOs determined by GC–MS are presented in
Table 1 and
Table 2. Nine chemical compounds were identified from basil EOs with the total percentage of 95.1%, in which methyl chavicol (61.3%) and linalool (24.8%) were the main components (
Table 1). This finding was in agreement with the study of Oxemham et al. [
19], Grayer et al. [
20], Marotti et al. [
21], and Sishu et al. [
22]. Basil varieties could be classified into two groups on the basis of EO chemotype: methyl chavicol and linalool [
19]. Methyl chavicol and linalool chemotye contain methyl chavicol and linalool, respectively, as the most abundant component in EOs [
19,
20,
21]. Basil EOs in this study could be considered as methyl chavicol chemotype. In the study of Oxemham et al. [
19], the methyl chavicol chemotype contained methyl chavicol (76.1%) and linalol (18.6%), while linalol chemotype had linalol, eugenol, eucalyptol, and caryophyllene of 53%, 12.4%, 7.7%, and 5% of the whole oil, respectively.
A total of 13 chemical components accounting for 89.1% of cinnamon bark EOs were determined (
Table 1). Trans-cinnamaldehyde (43.3%), safrole (26.7%), cis-cinnamaldehyde (10.9%), and 1,8-cineole (4.5%) were the main constituents of EOs. Commercial cinnamon EOs are commonly extracted from the bark of three cultivated species, namely,
Cinnamomum cassia,
C. loureirii, and
C. verum synonym
C. zeylanicum [
23]. Bark EOs of
C. cassia have trans-cinnamaldehyde (42.4–90.6%), cinnamic acid (5.5–43.1%), methoxy-cinnamaldehyde (3.5–13.8%), and cynnamyl acetate (1.0–5.4%) as the main compositions [
24,
25,
26,
27,
28,
29,
30].
C. loureirii bark EOs commonly contains four main components, namely, trans-cinnamaldehyde (50.2–92.9%), α-copaene (2.1–21.3%), α-guaiene (3.4–9.3%), and β-cadinene (4.1–7.7%) [
31,
32,
33,
34,
35,
36].
C. verum varieties are grouped into two chemotypes according to their main components in EOs: eugenol and safrole chemotype [
23,
36]. Bark EOs of eugenol chemotype usually have four marker compounds, namely, trans-cinnaldehyde (50.5–89.3%), cinnamyl acetate (1.5–8.8%), eugenol (0.4–8.8%), and 1,8-cineole (1.0–4.6%) [
24,
32,
34,
37,
38]. Safrole chemotype often contains two fingerprint compounds in bark EOs, namely, trans-cinnamaldehyde (52.5–74%) and safrole (2–10.8%) [
23,
39]. In addition, other species from
Cinnomomum genus (
C. pubescens,
C. impressicostatum,
C. mollissimum,
C. porrectum, and
C. camphora) have a high content of safrole (3.2–93.4%) in their bark EOs; however, they have no content of trans-cinnamaldehyde [
40]. From the literature review of EOs extracted from barks of cinnamon species, it can be suggested that cinnamon EOs in this study with two main marker compounds identified (trans-cinnamaldehyde and safrole) belong to the safrole chemotype of
C. verum. There were 16 chemical compounds determined in lemongrass EOs, accounting for 87.3% of the total oil content (
Table 1). Geranial (34.6%), neral (34.5%), geraniol (3.4%), geranyl acetate (2.6%), and cis-carveol (2.0%) were the main components of lemongrass EOs. The results were in agreement with the chemical composition of
Cymbopogon citratus EOs reported in several studies [
3,
41,
42,
43,
44]. A natural mixture of two isomeric acyclic monoterpene aldehydes, geranial (transcitral, citral A) and neral (cis-citral, citral B), is named as citral, being the main chemical component of lemongrass oil. Geranial and neral have the same molecular formula, C
10H
16O, but have differently chemical structures.
EOs extracted from the fruit peels of “Cam Sanh” orange had eight chemical compounds identified with a total of 88.7% (
Table 2). Limonene (87.2%) and β-myrcene (0.9%) were the main components of “Cam Sanh” orange peel oil. Cam Sanh orange is the hybrid species of
Citrus reticulata and
Citrus sinensis, with the scientific name of
Citrus reticulata x sinensis. There is very limited information reported in the literature about the chemical composition of EOs extracted from this hybrid. However, EOs from fruit peels of
C. reticulata and
C. sinensis have been extensively documented.
C. reticulata oils have four main chemical components, namely, limonene (65.3–74.2%), γ-terpinene (16.4–22.7%), α-pinene (2.0–2.7%), and β-pinene (1.4–2.1%), while
C. sinensis oils have limonene (83.9–95.9%) and β-myrcene (0.9–3.3%) as the main components [
3]. It can be seen that the chemical composition of Cam Sanh orange peel EOs is closely related to that of
C. sinensis.A total of 12 chemical compounds identified from peppermint (
Mentha piperita) EOs accounted for 92.0% of oil content (
Table 2). Carvone (61.6%), pulegone (12.3%), and limonene (6.2%) were the main compositions of peppermint oil. Peppermint EOs can be sorted into two chemotypes according to their content of carvone and menthol. Carvone chemotype contains three main compounds, namely, carvone (34.9–84.3%), pulegone (0.8–14.8%), and limonene (8.1–11.2%), while menthol chemotype has three different dominant compounds, namely, menthol (22.3–54.2%), menthone (9.1–39.3%), and menthyl acetate (2.0–15.1%) [
45,
46,
47,
48,
49,
50]. Consequently, peppermint oil in this study may belong to carvone chemotype.
Coriander (
Coriandrum sativum) fruit EOs had 21 chemical compounds identified, with a total of 74.9% oil content (
Table 2). Linalool (55.3%), neryl acetate (4.3%), γ-terpinene (3.1%), and p-cymene (2.6%) were the main constituents of coriander fruit oil. The content of these compounds was within the content range reported in the literature [
3,
51,
52,
53,
54] regarding coriander fruit EOs with linalool (36.7–87.5%), neryl acetate (0–8.4%), γ-terpinene (0.1–9.1%), and p-cymene (2.6%).
The chemical compositions of EOs extracted from the same part of the same plant species are not the same across the different studies reported in the literature. This could be due to the fact that the chemical constituents of plant EOs are greatly affected by five main factors: genetics (varieties), environment (geographical, climatic, seasonal, and soil conditions of growing location), cultivation (irrigation, fertilization, harvesting time, stage of maturity), sample preparation (drying methods, fresh or dry materials, particle size of plant samples), and extraction methods (solvent extraction, hydro-distillation, CO2 extraction, microwave-assisted extraction).
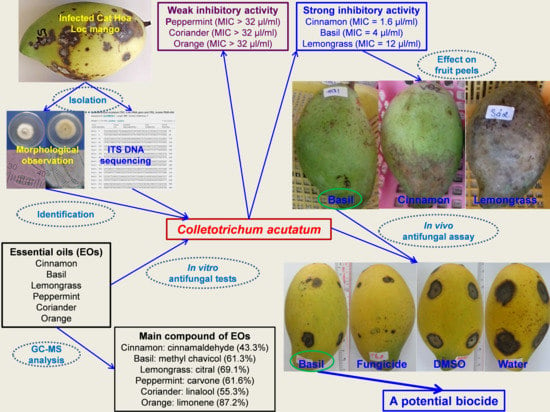
3.2. Isolation of the Most Pathogenic Fungus
The isolation of the fungal strains causing anthracnose disease on the fruits of Cat Hoa Loc variety was based on the disease lesions described in the study of Le Hoang Le Thuy and Pham Van Kim [
55]. During the process of collecting the infected fruits, the disease symptoms were mainly observed on young leaves, flowers, and fruits. The initial symptoms were from small brown to black spots. There were many disease spots on the leaves, and these spots joined together to form large, brown to black lesions, surrounded by dark brown margins. Young leaves could not develop and consequently affect plant growth. The infected flowers were black and could not bear fruit. The diseased fruits had irregular black spots on the peel that caused fruit drop or affected post-harvest fruit quality. From over 10 fungal strains isolated from the infected fruits, four strains (namely, Col 1, Col 2, Col 5, and Col 6) were artificially demonstrated to cause anthracnose on mango fruits, showing dark brown and black lesions.
Four pathogenic strains were compared to select the strain that caused the most damage to mango fruits. The diameter of disease lesions on Cat Hoa Loc mango fruits caused by four fungal strains that was measured at 6, 9, 12, and 15 days after artificial infection (D.A.I.) is shown in
Table 3. Initially, most of the lesions were round, while a few were irregular shape. Subsequently, they were dry with dark brown color and slightly concave, having no discernible border (
Figure 1). At 6 D.A.I., all fungal strains showed pathogenicity with lesion diameter ranging from 1.8 to 9.9 mm, in which Col 5 strain induced the largest diameter of 9.9 mm. The damage caused by four fungal strains on mango fruits increased over time. At 15 D.A.I., the strains of Col 1, Col 5, and Col 6 showed the highest diameters of 19.2, 20.0, and 16.2 mm, respectively, with there being no statistically significant difference. It can be seen that the Col 5 strain caused the most damage to mango fruits as compared to the remaining strains (
Table 3,
Figure 1). Therefore, it was selected for the identification and use in the following experiments.
3.3. Identification of the Most Pathogenic Fungus
The cultural and morphological characteristics of the studied fungus are presented in
Figure 2. The colony produced the aerial mycelium with a smooth filamentous form and even edge. The mycelial growth was relatively slow—after 9 days of inoculation, the mycelial diameter in the Petri dish was about 5.5 cm. Mycelium of
C. acutatum reached the edge of the Petri dish at about 14 days, while the mycelial growth rate of
C. gloeosporioides was relatively fast and reached the edge of the Petri dish at about 10 days [
56]. The upper colony surface was white, while the lower colony surface had a light orange color. The fungal conidia were fusiform and cylindrical with an acute end. Conidiophores were mostly simple, sometimes branched. The characteristics of the studied isolate causing anthracnose on mango in this study were consistent with the description of Sutton [
13] regarding
Colletotrichum spp. Furthermore, these characteristics were similar to those of
Colletotrichum acutatum described in the studies of Khan et al. [
56], Jayasinghe et al. [
57], Sundelin et al. [
58], and Peres et al. [
59].
The rDNA sequence of ITS1 and ITS2 regions including the 5.8S ribosomal subunit of the isolate was successfully amplified with a length of 586 nucleotides by using the universal primer pair of
ITS1 and
ITS4. The similar length of this region was also reported in the study of Shi et al. [
60], who amplified DNA extracted from
Colletotrichum acutatum by using
ITS1 and
ITS4 primers. BLAST similarity search showed that the sequence of ITS1, 5.8S rRNA, and ITS2 from the studied isolate shared the sequence identity of 99% with the published ITS sequences of
Colletotrichum acutatum in Genbank, with the accession number of AJ749675.1.
From the cultural, morphological, and molecular biological evidence presented above, we can conclude that the studied isolate causing anthracnose disease on Cat Hoa Loc mango fruits is
Colletotrichum acutatum. This fungus is one of the most frequently reported species of the genus and causes diseases commonly known as anthracnose on numerous host plants worldwide. It has been known to be especially destructive on fruits such as papaya and capsicum, strawberry, citrus, apple, olive, cranberry, blueberry, and mango. This fungal species has been demonstrated to be the most infectious, because it is resistant to a wide range of fungicides, namely, carbendazim [
57], benomyl and copper oxide [
59], quinone outside inhibitors [
61], and mancozeb and thiophanate-methyl [
62].
3.4. In Vitro Antifungal Activity of EOs against C. acutatum
The antifungal activity of six EOs assayed by the agar diffusion method is shown in
Table 4 and
Figure 3. A total of 10 µL of EO solution in DMSO with a concentration of 20 µL/mL or control (100% DMSO or 625 µg/mL Talent 50 WP) was tested against mycelial growth of
C. acutatum on an agar plate. The antifungal activity was determined by measuring the diameter of clear inhibitory zones appearing against a white background on the agar plate. These clear zones represented regions where fungal mycelia or reproductive stroma were not present [
63]. Among the tested EOs, the mean fungal growth inhibition of cinnamon bark EOs was the largest over 4 D.A.I., followed in descending order of basil, lemongrass, peppermint, and coriander seed oils. Orange peel oil showed no antifungal activity against
C. acutatum. Similarly, DMSO treatment had no growth inhibition recorded over 4 D.A.I., indicating that DMSO did not contribute to the antifungal activity of EO solution in DMSO.
The antifungal activity of all tested EOs was greatly reduced over 4 D.A.I., while that of prochloraz was almost unchanged in the same period. Across 4 days of observation, at the first D.A.I., the growth inhibition of all EOs and fungicide was the largest; however, at the second D.A.I., the reduction in inhibition was observed in all treatments (except orange EOs and DMSO treatment). At the third and fourth D.A.I., the antifungal activity of fungicide was unchanged as compared to that at the second DAI; however, all EO treatments showed significant decrease over the same period. Similar results were also found in the study of Morkeliūnė et al. [
64] in that the inhibitory activity of sage and peppermint EOs against
C. acutatum was lower at 7 D.A.I. as compared to that of 4 D.A.I. In the agar diffusion assay, EOs or fungicide solution was added on the filter paper placed onto the agar disc that had the surface completely covered by the fungal spore suspension. EOs and fungicide gradually diffused away from the filter paper. The further distance from the filter paper, the less concentration of EOs and fungicide. At the first D.A.I., not many fungal spores germinated, leading to low mycelial growth; therefore, EOs and fungicide at low concentration was very effective, resulting in the largest growth inhibition recorded in all treatments. However, at the second D.A.I., the mycelia development was vigorous, and EOs and fungicide became ineffective at low concentrations, leading to the reduction in the fungal growth inhibition. Beyond this date, further reduction was observed in all EO treatments, but not in fungicide treatment. It can be explained by the fact that EOs are volatile while prochloraz is non-volatile [
65]. Over time, the chemical components of EOs slowly escaped into the air, resulting in lower concentrations of EOs in agar disc, and therefore EOs treatments had less of an inhibitory effect on the mycelial growth. On the other hand, the unchanged concentration of fungicide in agar disc owing to its non-volatile property maintained the effectively inhibitory activity against the fungal development.
MIC of six EOs against
C. acutatum is presented in
Table 5. The lower the MIC value of EOs, the higher the antifungal activity. MIC of cinnamon bark EOs was the lowest (1.6 µL/mL), followed by basil (4 µL/mL) and lemongrass (12 mL) EOs. MIC of orange, peppermint, and coriander EOs was greater than 32 µL/mL. The antifungal activity of EOs expressed by MIC was consistent with that indicated by the fungal growth inhibition in
Table 4. Cinnamon bark EOs had the strongest antifungal activity, followed in descending order by basil, lemongrass, peppermint, coriander, and orange EOs. Similarly, the antifungal activity against
C. acutatum causing grape ripe rot of cinnamon (
Cinnamomum cassia) EOs was the strongest as compared to that of holy basil and peppermint EOs [
66]. The study of Morkeliūnė et al. [
64] also reported that peppermint and coriander EOs had weak antifungal activity against
C. acutatum, causing anthracnose on strawberry fruits.
The antifungal activity of cinnamon EOs may be primarily related to their major components. The major constituents of cinnamon EOs were found to be trans-cinnamaldehyde (43.3%), safrole (26.7%), cis-cinnamaldehyde (10.9%), and 1,8-cineole (4.5%) (
Table 1). Trans-cinnamaldehyde was demonstrated to be a potent antifungal agent [
67,
68,
69]. Safrole was shown to possess a moderate inhibitory activity against
C. acutatum isolated from the infected tamarillo. 1,8-Cineole had no inhibitory activity against
C. acutatum isolated from ripe rot diseased grapes [
70]. Therefore, the antifungal activity of cinnamon bark oil may be mainly due to trans-cinnamaldehyde. The higher the trans-cinnamaldehyde content in cinnamon oil, the stronger the antifungal activity of cinnamon oil expressed by its lower MIC against
C. acutatum. It is further supported by comparing MIC and trans-cinnamaldehyde content of cinnamon oil in this study with those reported in the study of Duduk et al. [
71] and He et al. [
72]. MIC of cinnamon oil in this study was the highest (MIC = 1.6 µL/mL), while its trans-cinnamaldehyde content was the lowest (43.3%). In contrast, cinnamon bark oil had the lowest MIC (0.2 µL/mL) against
C. acutatum, causing anthracnose on kiwifruit, with the highest cinnamaldehyde content (86.2%) [
72]. Cinnamon bark oil had the moderate MIC (0.67 µL/mL) against
C. acutatum causing anthracnose on strawberry fruits and moderate trans-cinnamaldehyde content (73%) [
71].
The strong antifungal activity of basil oil may be due to its main compositions, namely, methyl chavicol (61.3%) and linalool (24.8%) (
Table 1). Methyl chavicol was proven to be a strong antifungal agent against
Moniliophthora perniciosa with minimum fungicidal concentration (MFC) of 1000 ppm [
73] and
Uromyces viciae-fabae with MFC of 1000 ppm [
19]. Similarly, linalool showed the strong inhibitory activity against
Uromyces viciae-fabae with MFC of 1000 ppm [
19]. MFC is defined as the concentration of EOs, resulting in the death of 99.9% of the fungi. Methyl chavicol and linalool significantly reduced the mycelial growth of
Botrytis fabae on solid media at concentrations of 1000 and 600 ppm, respectively, at 4 days after inoculation [
19]. However, linalool was demonstrated to be ineffective in controlling the growth of three
Colletotrichum species, namely,
C. acutatum, C. fructicola, and
C. gloeosporioides [
67,
70]. Consequently, the antifungal activity of basil oil against
C. acutatum in this study may be primarily from methyl chavicol.
Lemongrass oil had strong antifungal activity against
C. acutatum due to its citral content (69.1%) (
Table 1). Citral is a strong antifungal agent against many species of plant pathogenic fungi. It completely inhibited the mycelial growth of
Colleotrichum fructicola and
Colleotrichum acutatum isolated from ripe rot diseased grapes at concentration of 1 mg/mL [
70]. Furthermore, MIC of citral against
Colletotrichum musae,
Colletotrichum gloeosporioides, and
Fusarium subglutinans was very low (0.6%) [
74]. Volatile citral completely suppressed the conidial germination of
C. gloeosporioides isolated from the infected pepper fruit at the concentration of 2 µL/disc [
67]. Citral in the vapor form totally controlled the mycelial growth of
Colletotrichum lindemuthianum,
Fusarium oxysporum, and
Botrytis cinerea at a very low concentration of 10 ng/mL in the atmosphere [
75].
Peppermint, coriander, and orange EOs had negligible or no antifungal activity against
C. acutatum (
Table 4 and
Table 5). It may be due to the fact that their main components have low antifungal activity. Carvone (61.6%), linalool (55.3%), and limonene (87.2%) were the main components of peppermint, coriander, and orange EOs, respectively (
Table 2). Carvone was not effective against
Fusarium subglutinans but had low inhibitory activity against
C. musae and
C. gloeosporioides with MICs of 0.8 and 1%, respectively [
74]. Linalool and limonene were ineffective in inhibiting the mycelial growth of
C. fructicola and
C. acutatum isolated from ripe rot diseased grapes at a concentration of 1 mg/mL [
70]. In the vapor form, carvone and limonene had low antifungal activity against the mycelial growth of
C. lindemuthianum,
F. oxysporum, and
B. cinerea [
75]. Volatile linalool and limonene could not completely suppress the conidial germination of
C. gloeosporioides isolated from the infected pepper fruit at the concentration of 8 µL/disc [
67].