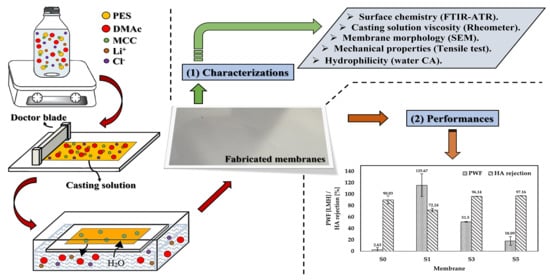
Microcrystalline Cellulose-Blended Polyethersulfone Membranes for Enhanced Water Permeability and Humic Acid Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MCC Solution
2.3. Membrane Fabrication
2.4. Preparation of HA Solution
2.5. Characterization
2.5.1. Viscosity of Casting Solutions
2.5.2. Membrane Morphology
2.5.3. Membrane Surface Chemistry
2.5.4. Membrane Mechanical Properties
2.5.5. Membrane Water Contact Angle
2.6. Membrane Flux and HA Rejection Evaluation
3. Results and Discussion
3.1. Surface Functional Groups
3.2. Casting Solution Viscosity
3.3. Membranes Morphology and Dope Viscosity
3.4. Mechanical Properties of Fabricated Membranes
3.5. Hydrophilicity Analysis of Fabricated Membranes
3.6. Permeability and Separation Performance of Fabricated Membranes
3.7. Comparison with Other Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AMTEC. Membrane Technology: A Sustainable Environmental and Industrial Solution. Available online: https://www.utm.my/amtec/?page_id=641 (accessed on 8 December 2020).
- Yang, M.; Hadi, P.; Yin, X.; Yu, J.; Huang, X.; Ma, H.; Walker, H.; Hsiao, B.S. Antifouling nanocellulose membranes: How subtle adjustment of surface charge lead to self-cleaning property. J. Membr. Sci. 2021, 618. [Google Scholar] [CrossRef]
- Athira, V.B.; Mohanty, S.; Nayak, S.K. Preparation and characterization of porous polyethersulfone (PES) membranes with improved biocompatibility by blending sulfonated polyethersulfone (SPES) and cellulose acetate (CA)—A comparative study. Mater. Today Commun. 2020, 25, 101544. [Google Scholar] [CrossRef]
- Hudaib, B.; Gomes, V.; Shi, J.; Zhou, C.; Liu, Z. Poly (vinylidene fluoride)/polyaniline/MWCNT nanocomposite ultrafiltration membrane for natural organic matter removal. Sep Purif Technol 2018, 190, 143–155. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, S.; Song, J.; Zhao, Y.; Yang, F. Activation of porous magnetized biochar by artificial humic acid for effective removal of lead ions. J. Hazard. Mater. 2020, 389, 122115. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Fan, J.; He, T.; Xu, X.; Ai, Y.; Tang, H.; Gu, H.; Lu, T.; Liu, Y.; Liu, G. The mechanism of aquatic photodegradation of organophosphorus sensitized by humic acid-Fe3+ complexes. J. Hazard. Mater. 2020, 384, 121466. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Collado, S.; Oulego, P.; Díaz, M. The wet oxidation of aqueous humic acids. J. Hazard. Mater. 2020, 396, 122402. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Y.; Qu, G.; Sun, Q.; Liang, D.; Hu, S.; Zhu, L. Enhanced removal of humic acid from micro-polluted source water in a surface discharge plasma system coupled with activated carbon. Environ. Sci. Pollut. Res. Int. 2017, 24, 21591–21600. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, S.; Yuan, Y.; Lu, Q. Influence of Humic Acid Complexation with Metal Ions on Extracellular Electron Transfer Activity. Sci. Rep. 2015, 5, 17067. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Tobiason, J.E.; Huang, H. Water and humic acid transport in graphene-derived membrane: Mechanisms and implications to functional membrane design. J. Membr. Sci. 2020, 613, 118441. [Google Scholar] [CrossRef]
- Vijesh, A.M.; Arathi Krishnan, P.V.; Isloor, A.M.; Shyma, P.C. Fabrication of PPSU/PANI hollow fiber membranes for humic acid removal. Mater. Today Proc. 2020, 247. [Google Scholar] [CrossRef]
- Wang, L.; Han, C.; Nadagouda, M.N.; Dionysiou, D.D. An innovative zinc oxide-coated zeolite adsorbent for removal of humic acid. J. Hazard. Mater. 2016, 313, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imyim, A.; Prapalimrungsi, E. Humic acids removal from water by aminopropyl functionalized rice husk ash. J. Hazard. Mater. 2010, 184, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, R.S.; Isloor, A.M.; Prabhu, B.; Asiri, A.M.; Ismail, A.F. Removal of metal ions and humic acids through polyetherimide membrane with grafted bentonite clay. Sci. Rep. 2018, 8, 4665. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.F.; Liang, J.P.; Zhao, Z.L.; Yuan, H.; Qiao, J.J.; Xu, Q.N.; Wang, H.L.; Wang, W.C.; Yang, D.Z. Ultra-high synergetic intensity for humic acid removal by coupling bubble discharge with activated carbon. J. Hazard. Mater. 2020, 403, 123626. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Recent advances in hydrophilic modification and performance of polyethersulfone (PES) membrane via additive blending. RSC Adv. 2018, 8, 22710–22728. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, E.; Ng, L.Y.; Ang, W.L.; Chung, Y.T.; Rohani, R.; Mohammad, A.W. Enhancing Morphology and Separation Performance of Polyamide 6,6 Membranes By Minimal Incorporation of Silver Decorated Graphene Oxide Nanoparticles. Sci. Rep. 2019, 9, 1216. [Google Scholar] [CrossRef] [Green Version]
- Teow, Y.H.; Ooi, B.S.; Ahmad, A.L.; Lim, J.K. Investigation of Anti-fouling and UV-Cleaning Properties of PVDF/TiO2 Mixed-Matrix Membrane for Humic Acid Removal. Membranes 2021, 11, 16. [Google Scholar] [CrossRef]
- Thuyavan, Y.L.; Anantharaman, N.; Arthanareeswaran, G.; Ismail, A.F. Adsorptive Removal of Humic Acid by Zirconia Embedded in a Poly(ether sulfone) Membrane. Ind. Eng. Chem. Res. 2014, 53, 11355–11364. [Google Scholar] [CrossRef]
- Celik, E.; Park, H.; Choi, H.; Choi, H. Carbon nanotube blended polyethersulfone membranes for fouling control in water treatment. Water research 2011, 45, 274–282. [Google Scholar] [CrossRef]
- Seshasayee, M.S.; Yu, Z.; Arthanareeswaran, G.; Das, D.B. Preparation of nanoclay embedded polymeric membranes for the filtration of natural organic matter (NOM) in a circular crossflow filtration system. J. Water Process Eng. 2020, 37, 101408. [Google Scholar] [CrossRef]
- Jamalludin, M.R.; Harun, Z.; Hubadillah, S.K.; Basri, H.; Ismail, A.F.; Othman, M.H.D.; Shohur, M.F.; Yunos, M.Z. Antifouling polysulfone membranes blended with green SiO2 from rice husk ash (RHA) for humic acid separation. Chem. Eng. Res. Des. 2016, 114, 268–279. [Google Scholar] [CrossRef]
- Chai, P.V.; Choy, P.Y.; Teoh, W.C.; Mahmoudi, E.; Ang, W.L. Graphene oxide based mixed matrix membrane in the presence of eco-friendly natural additive gum Arabic. J. Environ. Chem. Eng. 2021, 9, 105638. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, W.; Wang, D.; Ni, X.; Han, G. Electrospun polyvinylidene fluoride-based fibrous nanocomposite membranes reinforced by cellulose nanocrystals for efficient separation of water-in-oil emulsions. J. Membr. Sci. 2019, 575, 71–79. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S. Polyethersulfone (PES)/cellulose acetate phthalate (CAP) blend ultrafiltration membranes: Preparation, morphology, performance and antifouling properties. J. Membr. Sci. 2007, 305, 299–312. [Google Scholar] [CrossRef]
- Zha, S.; Yu, J.; Zhang, G.; Liu, N.; Lee, R. Polyethersulfone/Cellulose Acetate Butyrate Hybrid Hollow-Fiber Membranes for Organic-Matter Removal From Produced Water. SPE J. 2017, 22, 1478–1486. [Google Scholar] [CrossRef]
- Evangeline, C.; Pragasam, V.; Rambabu, K.; Velu, S.; Monash, P.; Arthanareeswaran, G.; Banat, F.J.D.W.T. Iron oxide modified polyethersulfone/cellulose acetate blend membrane for enhanced defluoridation application. Desalination Water Treat. 2019, 156, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Wu, H.; Ding, J.; Ding, A.; Zhang, X.; Ren, N.; Li, G.; Liang, H. Cellulose nanocrystal-blended polyethersulfone membranes for enhanced removal of natural organic matter and alleviation of membrane fouling. Chem. Eng. J. 2020, 382, 122919. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Ding, A.; Ren, N.; Li, G.; Liang, H. Surface coating of UF membranes to improve antifouling properties: A comparison study between cellulose nanocrystals (CNCs) and cellulose nanofibrils (CNFs). Chemosphere 2019, 217, 76–84. [Google Scholar] [CrossRef]
- Ding, Z.; Zhong, L.; Wang, X.; Zhang, L. Effect of lignin–cellulose nanofibrils on the hydrophilicity and mechanical properties of polyethersulfone ultrafiltration membranes. High Perform. Polym. 2016, 28, 1192–1200. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Hui Chuin, C.T.; Sabar, S.; Fazita, M.R.; Taiwo, O.F.; Hassan, T.M.; Haafiz, M.K. Microcrystalline cellulose: Isolation, characterization and bio-composites application–A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Hindi, S.S.Z. Microcrystalline Cellulose: The Inexhaustible Treasure for Pharmaceutical Industry. J. Nanosci. Nanotechnol. Res. 2017, 4, 17–24. [Google Scholar] [CrossRef]
- Long, W.-J.; Tao, J.-L.; Lin, C.; Gu, Y.-c.; Mei, L.; Duan, H.-B.; Xing, F. Rheology and buildability of sustainable cement-based composites containing micro-crystalline cellulose for 3D-printing. J. Clean. Prod. 2019, 239, 118054. [Google Scholar] [CrossRef]
- Mohamad Haafiz, M.K.; Eichhorn, S.J.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.; Jawaid, M.; Karim, Z.; Abdullah, L.C. Morphological, Physiochemical and Thermal Properties of Microcrystalline Cellulose (MCC) Extracted from Bamboo Fiber. Molecules 2020, 25, 2824. [Google Scholar] [CrossRef]
- Hussin, M.H.; Pohan, N.A.; Garba, Z.N.; Kassim, M.J.; Rahim, A.A.; Brosse, N.; Yemloul, M.; Fazita, M.R.; Haafiz, M.K. Physicochemical of microcrystalline cellulose from oil palm fronds as potential methylene blue adsorbents. Int. J. Biol. Macromol. 2016, 92, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Rafieian, F.; Mousavi, M.; Dufresne, A.; Yu, Q. Polyethersulfone membrane embedded with amine functionalized microcrystalline cellulose. Int. J. Biol. Macromol. 2020, 164, 4444–4454. [Google Scholar] [CrossRef]
- El Hamdaoui, L.; El Bouchti, M.; El Moussaouiti, M. Comparison of rheological properties of kraft and microcrystalline cellulose dissolved in lithium chloride/N,N-dimethylacetamide. Polym. Bull. 2017, 75, 769–779. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Cai, X.; Yao, J.; Li, M.; Zhang, X.; Liu, Q. High alkaline tolerant electrolyte membrane with improved conductivity and mechanical strength via lithium chloride/dimethylacetamide dissolved microcrystalline cellulose for Zn-Air batteries. Electrochim. Acta 2016, 220, 635–642. [Google Scholar] [CrossRef]
- Han, N.; Zhang, W.; Wang, W.; Yang, C.; Tan, L.; Cui, Z.; Li, W.; Zhang, X. Amphiphilic cellulose for enhancing the antifouling and separation performances of poly (acrylonitrile-co-methyl acrylate) ultrafiltration membrane. J. Membr. Sci. 2019, 591, 117276. [Google Scholar] [CrossRef]
- Ramos, L.A.; Frollini, E.; Heinze, T. Carboxymethylation of cellulose in the new solvent dimethyl sulfoxide/tetrabutylammonium fluoride. Carbohydr. Polym. 2005, 60, 259–267. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Heiskanen, J.P. Room-temperature dissolution and chemical modification of cellulose in aqueous tetraethylammonium hydroxide–carbamide solutions. Cellulose 2019, 27, 1933–1950. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, D.; Xiao, H.; Zhang, H.; Cao, S.; Chen, L.; Ni, Y.; Huang, L. Ultra-low pressure cellulose-based nanofiltration membrane fabricated on layer-by-layer assembly for efficient sodium chloride removal. Carbohydr. Polym. 2020, 117352. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wu, Z.; Wei, Y.; Zhang, Y.; Yuan, Y.; Wang, J. Preparation of PVDF-CTFE hydrophobic membranes for MD application: Effect of LiCl-based mixed additives. J. Membr. Sci. 2016, 506, 71–85. [Google Scholar] [CrossRef]
- McCormick, C.L.; Callais, P.A.; Hutchinson Jr, B.H. Solution Studies of Cellulose in Lithium Chloride and N,N-Dimethylacetamide. Macromolecules 1985, 18, 2394–2401. [Google Scholar] [CrossRef]
- Morgenstern, B.; Kammer, H.W.; Berger, W.; Skrabal, P. 7Li-NMR study on cellulose/LiCl/N.N-dimethylacetamide solutions. Acta Polym. 1992, 43, 356–357. [Google Scholar] [CrossRef]
- Wan, Y.; An, F.; Zhou, P.; Li, Y.; Liu, Y.; Lu, C.; Chen, H. Regenerated cellulose I from LiCl.DMAc solution. Chem. Commun. 2017, 53, 3595–3597. [Google Scholar] [CrossRef]
- Zhang, D.; Karkooti, A.; Liu, L.; Sadrzadeh, M.; Thundat, T.; Liu, Y.; Narain, R. Fabrication of antifouling and antibacterial polyethersulfone (PES)/cellulose nanocrystals (CNC) nanocomposite membranes. J. Membr. Sci. 2018, 549, 350–356. [Google Scholar] [CrossRef]
- Chang, H.; Qu, F.; Liu, B.; Yu, H.; Li, K.; Shao, S.; Li, G.; Liang, H. Hydraulic irreversibility of ultrafiltration membrane fouling by humic acid: Effects of membrane properties and backwash water composition. J. Membr. Sci. 2015, 493, 723–733. [Google Scholar] [CrossRef]
- Nazri, A.I.; Ahmad, A.L.; Hussin, M.H. Fabrication of Microcrystalline Cellulose Incorporated Polyethersulfone Hybrid Mixed Matrix Membrane for Humic Acid Removal. J. Phys. Sci. 2019, 30, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Thiangtham, S.; Runt, J.; Saito, N.; Manuspiya, H. Fabrication of biocomposite membrane with microcrystalline cellulose (MCC) extracted from sugarcane bagasse by phase inversion method. Cellulose 2019, 27, 1367–1384. [Google Scholar] [CrossRef]
- Qi, G.; Xiong, L.; Wang, B.; Lin, X.; Zhang, H.; Li, H.; Huang, C.; Chen, X.; Wang, C.; Chen, X. Improvement and Characterization in Enzymatic Hydrolysis of Regenerated Wheat Straw Dissolved by LiCl/DMAc Solvent System. Appl. Biochem. Biotechnol. 2017, 181, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, R.; Xiang, J.; Kang, H.; Liu, Z.; Huang, Y. Dissolution mechanism of cellulose in N,N-dimethylacetamide/lithium chloride: Revisiting through molecular interactions. J. Phys. Chem. B 2014, 118, 9507–9514. [Google Scholar] [CrossRef]
- Mohd Hir, Z.A.; Abdullah, A.H.; Zainal, Z.; Lim, H.N. Visible light-active hybrid film photocatalyst of polyethersulfone–reduced TiO2: Photocatalytic response and radical trapping investigation. J. Mater. Sci. 2018, 53, 13264–13279. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F. Effect of LiCl concentration in the polymer dope on the structure and performance of hydrophobic PVDF hollow fiber membranes for CO2 absorption. Chem. Eng. J. 2010, 165, 980–988. [Google Scholar] [CrossRef]
- Zhang, Z.; An, Q.; Ji, Y.; Qian, J.; Gao, C. Effect of zero shear viscosity of the casting solution on the morphology and permeability of polysulfone membrane prepared via the phase-inversion process. Desalination 2010, 260, 43–50. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, J.; Yu, D.; Zhang, Y.; Wei, Y. Preparation of PVDF-CTFE hydrophobic membrane by non-solvent induced phase inversion: Relation between polymorphism and phase inversion. J. Membr. Sci. 2018, 550, 480–491. [Google Scholar] [CrossRef]
- Lessan, F.; Karimi, M.; Bañuelos, J.L.; Foudazi, R. Phase separation and performance of polyethersulfone/cellulose nanocrystals membranes. Polymer 2020, 186, 121969. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, G.; Zhang, H.; Yang, F. Exploration of permeability and antifouling performance on modified cellulose acetate ultrafiltration membrane with cellulose nanocrystals. Carbohydr. Polym. 2017, 174, 190–199. [Google Scholar] [CrossRef]
- Lalia, B.S.; Guillen, E.; Arafat, H.A.; Hashaikeh, R. Nanocrystalline cellulose reinforced PVDF-HFP membranes for membrane distillation application. Desalination 2014, 332, 134–141. [Google Scholar] [CrossRef]
- Kim, H.-C.; Dempsey, B.A. Membrane fouling due to alginate, SMP, EfOM, humic acid, and NOM. J. Membr. Sci. 2013, 428, 190–197. [Google Scholar] [CrossRef]









| Sample | PES 1 [g] | MCC 2 [g] | Co-Solvent | |
|---|---|---|---|---|
| LiCl 3 [g] | DMAc 4 [g] | |||
| S0 | 5.175 | 0 | 0 | 24.825 |
| S1 | 5.175 | 0.3 | 1.962 | 22.563 |
| S3 | 5.175 | 0.9 | 1.914 | 22.011 |
| S5 | 5.175 | 1.5 | 1.866 | 21.459 |
| Membrane | Tensile Strength [MPa] | Elongation-at-Break [%] |
|---|---|---|
| Pristine PES (S0) | 6.57 ± 1.52 | 11.88 ± 5.72 |
| PES/MCC (S3) | 5.71 ± 0.89 | 7.16 ± 1.71 |
| Membrane | Configuration | Operating Pressure | PWF 1 | Water Permeability 2 | Solute Rejection | Ref. | |
|---|---|---|---|---|---|---|---|
| Membrane | Filtration | ||||||
| PES/CA (18/2 wt.%) | FS | Dead-end | 2.94 bar | 15.00 LMH | Improved to 5.10 LMHB | Fluoride—8% | [28] |
| PES/CAP/PVP (12.8/3.2/2 wt.%) | FS | Crossflow | 3.45 bar | 400.00 LMH | Improved to 115.94 LMHB | Non-skim milk—99% | [26] |
| PES/CAB/PVP (27.26/1.74/1 wt.%) | HF | Inside-out | 6.89 bar | 2.60 LMH | Improved to 0.38 LMHB | Benzene—99.6% Toluene—98.3% Octanoic acid—99.6% Hexanoic acid—99.6% | [27] |
| PES/LCNF/PVP (18/0.3/1.2 wt.%) | FS | Dead-end | 1.00 bar | 692.30 LMH | Improved to 692.30 LMHB | BSA—95% | [31] |
| PES/CNC/PVP (15/0.15/2 wt.%) | FS | Dead-end | 2.78 bar | 190.00 LMH | Improved to 68.35 LMHB | BSA—96.2% | [49] |
| PES/CNC/PVP (15/2/3 wt.%) | FS | Dead-end | 0.60 bar | 291.00 LMH | Improved to 485.00 LMHB | HA—71.9% BSA—82.7% NaAlg—85% | [29] |
| PSf/GO/GA (18/2/3 wt.%) | FS | Dead-end | 4.00 bar | 58.68 LMH | Improved to 14.67 LMHB | HA—96.34% | [24] |
| PES/MCC (17.25/3 wt.%) | FS | Crossflow | 1.00 bar | 51.50 LMH | Improved to 51.50 LMHB | HA—96.14% | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazri, A.I.; Ahmad, A.L.; Hussin, M.H. Microcrystalline Cellulose-Blended Polyethersulfone Membranes for Enhanced Water Permeability and Humic Acid Removal. Membranes 2021, 11, 660. https://doi.org/10.3390/membranes11090660
Nazri AI, Ahmad AL, Hussin MH. Microcrystalline Cellulose-Blended Polyethersulfone Membranes for Enhanced Water Permeability and Humic Acid Removal. Membranes. 2021; 11(9):660. https://doi.org/10.3390/membranes11090660
Chicago/Turabian StyleNazri, Amirul Islah, Abdul Latif Ahmad, and Mohd Hazwan Hussin. 2021. "Microcrystalline Cellulose-Blended Polyethersulfone Membranes for Enhanced Water Permeability and Humic Acid Removal" Membranes 11, no. 9: 660. https://doi.org/10.3390/membranes11090660