S-Layer Ultrafiltration Membranes
Abstract
:1. Introduction
2. Ultrastructure and Self-Assembly of S-Layer Proteins
3. S-Layers as Molecular Sieves
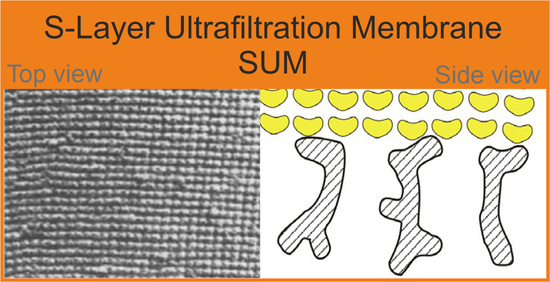
4. S-Layer Ultrafiltration Membrane (SUM)
5. S-Layers as Matrix for the Immobilization of Functional Macromolecules
6. SUM as Supporting Scaffold for Functional Lipid Membranes
7. Conclusions and Outlook
8. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavanasam, A.; Abbas, A. Ultrafiltration and Virus Removal: A Mini Review of Recent Patents. Recent Pat. Chem. Eng. 2008, 1, 151–156. [Google Scholar] [CrossRef]
- Pabby, A.K.; Rizvi, S.S.; Requena, A.M. (Eds.) Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Strathmann, H. Membrane separation processes. J. Membr. Sci. 1981, 9, 121–189. [Google Scholar] [CrossRef]
- Fane, A.; Fell, C.; Waters, A. The relationship between membrane surface pore characteristics and flux for ultrafiltration membranes. J. Membr. Sci. 1981, 9, 245–262. [Google Scholar] [CrossRef]
- Crowther, R.; Sleytr, U.B. An analysis of the fine structure of the surface layers from two strains of Clostridia, including correction for distorted images. J. Ultrastruct. Res. 1977, 58, 41–49. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Schuster, B.; Egelseer, E.M.; Pum, D. S-layers: Principles and applications. FEMS Microbiol. Rev. 2014, 38, 823–864. [Google Scholar] [CrossRef]
- Sára, M.; Sleytr, U.B. Verwendung isoporer, kristalliner Bakterienzellwandschichten als Ultrafiltrationsmembranen. Lebensmittel Biotechnol. 1985, 4, 141–146. [Google Scholar]
- Sleytr, U.B.; Sára, M. Ultrafiltration membranes with uniform pores from crystalline bacterial cell envelope layers. Appl. Microbiol. Biotechnol. 1986, 25, 83–90. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Sára, M. Herstellung isoporer Ultrafiltrationsmembranen aus kristallinen Bakterienzellwandschichten, In Tech-nische Membranen in der Biotechnologie; Kula, M.-R., Schügerl, K., Wandrey, C., Eds.; GBF Monographien, Bd. 9; Verlag Chemie: Weinheim, Germany, 1986; pp. 225–229. [Google Scholar]
- Sára, M.; Sleytr, U.B. Production and characteristics of ultrafiltration membranes with uniform pores from two-dimensional arrays of proteins. J. Membr. Sci. 1987, 33, 27–49. [Google Scholar] [CrossRef]
- Sára, M.; Manigley, C.; Wolf, G.; Sleytr, U.B. Isoporous ultrafiltration membranes from bacterial cell envelope layers. J. Membr. Sci. 1988, 36, 179–186. [Google Scholar] [CrossRef]
- Sára, M.; Sleytr, U.B. Membrane Biotechnology: Two-dimensional Protein Crystals for Ultrafiltration Purposes. In Biotechnology; Rehm, H.J., Ed.; VCH: Weinheim, Germany, 1988; Volume 6b, pp. 615–636. [Google Scholar]
- Sleytr, U.B.; Sára, M.; Wolf, G.; Manigley, C. Isoporous ultrafiltration membranes from crystalline cell envelope layers. In The Influence of New Technology on Medical Practice; Paul, J.P., McCruden, A.B., Schuetz, P.W., Eds.; Macmillan Press: Strathclyde, UK, 1988; pp. 83–87. [Google Scholar]
- Manigley, C.; Wolf, G.; Sára, M.; Sleytr, U.B. Comparative studies on synthetic and S layer ultrafiltration membranes. In Crystalline Bacterial Cell Surface Layers; Sleytr, U.B., Messner, P., Pum, D., Sára, M., Eds.; Springer Verlag: Berlin, Germany, 1988; pp. 154–159. [Google Scholar]
- Sára, M.; Wolf, G.; Küpcü, S.; Pum, D.; Sleytr, U.B. Use of crystalline bacterial cell envelope layers as ultrafiltration membranes and supports for the immobilization of macromolecules. In Dechema Biotechnology Conferences; VCH: Weinheim, Germany, 1988; Volume 2, pp. 35–51. [Google Scholar]
- Sára, M.; Küpcü, S.; Sleytr, U.B. Crystalline bacterial cell surface layers used as ultrafiltration membranes and immobilization matrix. Gen. Eng. Biotechnol. 1990, 10, 10–13. [Google Scholar]
- Küpcü, S.; Sára, M.; Sleytr, U.B. Chemical modification of crystalline ultrafiltration membranes and immobilization of macromolecules. J. Membr. Sci. 1991, 61, 167–175. [Google Scholar] [CrossRef]
- Sára, M.; Küpcü, S.; Weiner, C.; Weigert, S.; Sleytr, U.B. Crystalline protein layers as isoporous molecular sieves and immobi-lisation and affinity matrices. In Immobilised Macromolecules: Application Potentials; Sleytr, U.B., Messner, P., Pum, D., Sára, M., Eds.; Springer: London, UK, 1993; pp. 71–86. [Google Scholar]
- Weigert, S.; Sára, M. Surface modification of an ultrafiltration membrane with crystalline structure and studies on interactions with selected protein molecules. J. Membr. Sci. 1995, 106, 147–159. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Messner, P.; Pum, D.; Sára, M. Crystalline Bacterial Cell Surface Layers (S Layers): From Supramolecular Cell Structure to Biomimetics and Nanotechnology. Angew. Chem. Int. Ed. 1999, 38, 1034–1054. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Glauert, A.M. Analysis of regular arrays of subunits on bacterial surfaces: Evidence for a dynamic process of as-sembly. J. Ultrastruct. Res. 1975, 50, 103–116. [Google Scholar] [CrossRef]
- Sleytr, U.B. Regular Arrays of Macromolecules on Bacterial Cell Walls: Structure, Chemistry, Assembly, and Function. Adv. Clin. Chem. 1978, 53, 1–64. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Messner, P. Self-assembly of crystalline bacterial cell surface layers (S-layers). In Electron Microscopy of Subcellular Dynamics; Plattner, H., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 13–31. [Google Scholar]
- Messner, P.; Sleytr, U.B. The use of freeze-etching and freeze-drying to evaluate crystalline cell surface layers (S-layers). In Microbial Cell Surface Analysis Structural and Physicochemical Methods; Mozes, N., Handley, P.S., Busscher, H.J., Rouxhet, P.G., Eds.; VCH Publishers: New York, NY, USA, 1991; pp. 109–125. [Google Scholar]
- Pum, D.; Messner, P.; Sleytr, U.B. Role of the S layer in morphogenesis and cell division of the archaebacterium Methanocorpusculum sinense. J. Bacteriol. 1991, 173, 6865–6873. [Google Scholar] [CrossRef] [Green Version]
- Rachel, R. Cell envelopes of crenarchaeota and nanoarchaeota. In Prokaryotic Cell Wall Compounds—Structure and Biochemistry; König, H., Claus, H., Varma, A., Eds.; Springer: Heidelberg, Germany, 2010; Volume 9, pp. 271–291. [Google Scholar]
- Pavkov-Keller, T.; Howorka, S.; Keller, W. The Structure of Bacterial S-Layer Proteins. In Progress in Molecular Biology and Translational Science; Horworka, S., Ed.; Elsevier BV, Academic Press: Burlington, VT, USA, 2011; Volume 103, pp. 73–130. [Google Scholar]
- Thornley, M.J.; Glauert, A.M.; Sleytr, U.B. Structure and assembly of bacterial surface layers composed of regular arrays of subunits. Philos. Trans. R. Soc. B Biol. Sci. 1974, 268, 147–153. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Messner, P. Crystalline Surface Layers on Bacteria. Annu. Rev. Microbiol. 1983, 37, 311–339. [Google Scholar] [CrossRef]
- Baumeister, W.; Engelhardt, H. Three-Dimensional Structure of Bacterial Surface Layers; Harris, J.R., Horne, R.W., Eds.; Academic Press, Inc.: London, UK, 1987; Volume 6, pp. 109–154. [Google Scholar]
- Sleytr, U.B.; Messner, P.; Pum, D. 2 Analysis of Crystalline Bacterial Surface Layers by Freeze-etching, Metal Shadowing, Negative Staining and Ultrathin Sectioning. Methods Microbiol. 1988, 20, 29–60. [Google Scholar] [CrossRef]
- Beveridge, T.J. Bacterial S-layers. Curr. Opin. Struct. Biol. 1994, 4, 204–212. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Messner, P.; Pum, D.; Sára, M. Crystalline surface layers on eubacteria and archaeobacteria. In Crystalline Bacterial Cell Surface Proteins; Sleytr, U.B., Messner, P., Pum, D., Sára, M., Eds.; R.G. Landes Company and Academic Press, Inc.: Austin, TX, USA, 1996; pp. 211–225. [Google Scholar]
- Chung, S.; Shin, S.-H.; Bertozzi, C.R.; De Yoreo, J.J. Self-catalyzed growth of S layers via an amorphous-to-crystalline transition limited by folding kinetics. Proc. Natl. Acad. Sci. USA 2010, 107, 16536–16541. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.J.; Baumeister, W.; Engel, A. Conformational change of the hexagonally packed intermediate layer of Deinococcus radiodurans monitored by atomic force microscopy. J. Bacteriol. 1996, 178, 3025–3030. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.J.; Baumeister, W.; Engel, A. Controlled unzipping of a bacterial surface layer with atomic force microscopy. Proc. Natl. Acad. Sci. USA 1999, 96, 13170–13174. [Google Scholar] [CrossRef] [Green Version]
- Ebner, A.; Kienberger, F.; Huber, C.; Kamruzzahan, A.S.M.; Pastushenko, V.P.; Tang, J.; Kada, G.; Gruber, H.J.; Sleytr, U.B.; Sára, M.; et al. Atomic-Force-Microscopy Imaging and Molecular-Recognition-Force Microscopy of Recrystallized Heterotetramers Comprising an S-Layer-Streptavidin Fusion Protein. ChemBioChem 2006, 7, 588–591. [Google Scholar] [CrossRef]
- Moreno-Flores, S.; Kasry, A.; Butt, H.-J.; Vavilala, C.; Schmittel, M.; Pum, D.; Sleytr, U.B.; Toca-Herrera, J.L. From Native to Non-Native Two-Dimensional Protein Lattices through Underlying Hydrophilic/Hydrophobic Nanoprotrusions. Angew. Chem. Int. Ed. 2008, 47, 4707–4710. [Google Scholar] [CrossRef]
- Lopez, A.E.; Pum, D.; Sleytr, U.B.; Toca-Herrera, J.L. Influence of surface chemistry and protein concentration on the adsorption rate and S-layer crystal formation. Phys. Chem. Chem. Phys. 2011, 13, 11905–11913. [Google Scholar] [CrossRef]
- Sleytr, U.B. Basic and applied S-layer research: An overview. FEMS Microbiol. Rev. 1997, 20, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Sleytr, U.B.; Sára, M.; Pum, D.; Schuster, B. Characterization and use of crystalline bacterial cell surface layers. Prog. Surf. Sci. 2001, 68, 231–278. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Sára, M.; Pum, D.; Schuster, B. Molecular nanotechnology and nanobiotechnology with two-dimensional protein crystals (S-layers). In Nano-Surface Chemistry; Rosoff, M., Ed.; Marcel Dekker: New York, NY, USA; Basel, Switzerland, 2001; pp. 333–389. [Google Scholar]
- Sleytr, U.B.; Sára, M.; Pum, D.; Schuster, B.; Messner, P.; Schäffer, C. Self Assembly Protein Systems: Microbial S-Layers. In Biopolymers; Steinbüchel, A., Fahnenstock, S., Eds.; Wiley-VCH: Weinheim, Germany, 2002; Volume 7, pp. 285–338. [Google Scholar]
- Sleytr, U.B.; Sára, M.; Pum, D.; Schuster, B. Crystalline bacterial cell surface layers (S layers): A versatile self-assembly system. In Supramolecular Polymers, 2nd ed.; Ciferri, A., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, US, 2005; pp. 583–616. [Google Scholar]
- Schuster, B.; Sleytr, U.B. Nanotechnology with S-layer proteins. In Methods in Molecular Biology, Protein Nanotechnology, Pro-tocols, Instrumentation and Applications; Gerrard, J.A., Domigan, L.J., Eds.; Humana Press, Springer Science + Business Media: New York, NY, USA, 2020; Volume 2073, pp. 195–218. ISBN 978-1-4939-9868-5. [Google Scholar]
- Pum, D.; Sleytr, U.B. Reassembly of S-layer proteins. Nanotechnology 2014, 25, 312001. [Google Scholar] [CrossRef]
- Pum, D.; Toca-Herrera, J.L.; Sleytr, U.B. S-Layer Protein Self-Assembly. Int. J. Mol. Sci. 2013, 14, 2484–2501. [Google Scholar] [CrossRef]
- Sleytr, U.B. Heterologous reattachment of regular arrays of glycoproteins on bacterial surfaces. Nat. Cell Biol. 1975, 257, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, R.; Welsch, R.; Sára, M.; Sleytr, U.B. Stability and Self-Assembly of the S-Layer Protein of the Cell Wall of Bacillus stearothermophilus. Biol. Chem. Hoppe-Seyler 1985, 366, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Nomellini, J.F.; Küpcü, S.; Sleytr, U.B.; Smit, J. Factors controlling in vitro recrystallization of the Caulobacter crescentus paracrystalline S-layer. J. Bacteriol. 1997, 179, 6349–6354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, J.; Li, P.-N.; Jabbarpour, F.; Chan, A.C.K.; Rajkovic, I.; Matsui, T.; Shapiro, L.; Smit, J.; Weiss, T.M.; Murphy, M.E.P.; et al. A bacterial surface layer protein exploits multistep crystallization for rapid self-assembly. Proc. Natl. Acad. Sci. USA 2020, 117, 388–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comolli, L.R.; Siegerist, C.E.; Shin, S.-H.; Bertozzi, C.; Regan, W.; Zettl, A.; De Yoreo, J. Conformational Transitions at an S-Layer Growing Boundary Resolved by Cryo-TEM. Angew. Chem. Int. Ed. 2013, 52, 4829–4832. [Google Scholar] [CrossRef]
- Sára, M.; Sleytr, U.B. Molecular sieving through S layers of Bacillus stearothermophilus strains. J. Bacteriol. 1987, 169, 4092–4098. [Google Scholar] [CrossRef] [Green Version]
- Messner, P.; Schäffer, C.; Egelseer, E.M.; Sleytr, U.B. Occurrence, structure, chemistry, genetics, morphogenesis, and function of S-layers. In Prokaryotic Cell Wall Compounds—Structure and Biochemistry; König, H., Claus, H., Varma, A., Eds.; Springer: Heidelberg, Germany, 2010; Volume 2, pp. 53–109. [Google Scholar]
- Sára, M.; Sleytr, U.B. Relevance of charged groups for the integrity of the S-layer from Bacillus coagulans E38-66 and for molecular interactions. J. Bacteriol. 1993, 175, 2248–2254. [Google Scholar] [CrossRef] [Green Version]
- Sára, M.; Küpcü, S.; Sleytr, U.B. Biotechnological applications of S layers. In Crystalline Bacterial Cell Surface Layer Proteins (S Layers); Sleytr, U.B., Messner, P., Pum, D., Sára, M., Eds.; Academic Press, R.G. Landes Company: Austin, TX, USA, 1996; pp. 133–159. [Google Scholar]
- Weigert, S.; Sára, M. Ultrafiltration membranes prepared from crystalline bacterial cell surface layers as model systems for studying the influence of surface properties on protein adsorption. J. Membr. Sci. 1996, 121, 185–196. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Sára, M.; Küpcü, Z.; Messner, P. Structural and chemical characterization of S-layers of selected strains of Bacillus stearothermophilus and Desulfotomaculum nigrificans. Arch. Microbiol. 1986, 146, 19–24. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Sara, M. Structure with Membrane Having Continuous Pores. U.S. Patent 4,752,395, 21 June 1988. [Google Scholar]
- Sleytr, U.B.; Sara, M. Use of Structure with Membrane Having Continuous Pores. U.S. Patent 4,849,109, 18 July 1989. [Google Scholar]
- Sára, M.; Wolf, G.; Sleytr, U.B. Permeability properties and the use of S-layers for the production of ultrafiltration membranes, In Crystalline Bacterial Cell Surface Layers; Sleytr, U.B., Messner, P., Pum, D., Sára, M., Eds.; Springer: Berlin, Germany, 1988; pp. 149–153. [Google Scholar]
- Küpcü, S.; Sára, M.; Sleytr, U.B. Influence of covalent attachment of low molecular weight substances on the rejection and adsorption properties of crystalline proteinaceous ultrafiltration membranes. Desalination 1993, 90, 65–76. [Google Scholar] [CrossRef]
- Jim, K.; Fane, A.; Fell, C.; Joy, D.J. Fouling mechanisms of membranes during protein ultrafiltration. J. Membr. Sci. 1992, 68, 79–91. [Google Scholar] [CrossRef]
- Nilsson, J.L. Protein fouling of uf membranes: Causes and consequences. J. Membr. Sci. 1990, 52, 121–142. [Google Scholar] [CrossRef]
- Sára, M.; Pum, D.; Sleytr, U.B. Permeability and charge-dependent adsorption properties of the S-layer lattice from Bacillus coagulans E38-66. J. Bacteriol. 1992, 174, 3487–3493. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Flores, S.; Friedmann, J.; Pum, D.; Sleytr, U.B. Chemical and thermal denaturation of crystalline bacterial S-layer proteins: An atomic force microscopy study. Microsc. Res. Tech. 2004, 65, 226–234. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Sára, M.; Pum, D. Crystalline bacterial cell surface layers (S-layers): A versatile self-assembly system. In Supramolecular Polymerization; Ciferri, A., Ed.; Marcel Dekker: New York, NY, USA; Basel, Switzerland, 2000; pp. 177–213. [Google Scholar]
- Weiner, C.; Sára, M.; Dasgupta, G.; Sleytr, U.B. Affinity cross-flow filtration: Purification of IgG with a novel protein a affinity matrix prepared from two-dimensional protein crystals. Biotechnol. Bioeng. 1994, 44, 55–65. [Google Scholar] [CrossRef]
- Mosbach, K. Immobilized enzymes and cells. Struct. Order Polym. 1981, 136, 231–238. [Google Scholar] [CrossRef]
- Mosbach, K. Immobilized Enzymes and Cells Part D. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: Cambridge, MA, USA, 1988. [Google Scholar]
- Howorka, S.; Sára, M.; Wang, Y.; Kuen, B.; Sleytr, U.B.; Lubitz, W.; Bayley, H. Surface-accessible Residues in the Monomeric and Assembled Forms of a Bacterial Surface Layer Protein. J. Biol. Chem. 2000, 275, 37876–37886. [Google Scholar] [CrossRef] [Green Version]
- Sleytr, U.B.; Schuster, B.; Egelseer, E.M.; Pum, D.; Horejs, C.M.; Tscheliessnig, R.; Ilk, N. Nanotechnology with S-Layer Proteins as Building Blocks. In Progress in Molecular Biology and Translational Science; Horworka, S., Ed.; Elsevier, BV, Academic Press: Burlington, VT, USA, 2011; Volume 103, pp. 277–352. [Google Scholar]
- Sleytr, U.B.; Huber, C.; Ilk, N.; Pum, D.; Schuster, B.; Egelseer, E.M. S-layers as a tool kit for nanobiotechnological applications. FEMS Microbiol. Lett. 2007, 267, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Sleytr, U.B.; Pum, D.; Egelseer, E.M.; Ilk, N.; Schuster, B. S-Layer Proteins. In Handbook of Biofunctional Surfaces; Knoll, W., Ed.; Pan Stanford Publishing: Singapore, 2013; pp. 507–568. [Google Scholar] [CrossRef] [Green Version]
- Weiner, C.; Sára, M.; Sleytr, U.B. Novel protein a affinity matrix prepared from two-dimensional protein crystals. Biotechnol. Bioeng. 1994, 43, 321–330. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Sára, M. Use of regularly structured bacterial cell envelope layers as matrix for the immobilization of macromolecules. Appl. Microbiol. Biotechnol. 1989, 30, 184–189. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Sára, M. Introduction of sulphhydryl groups into the crystalline bacterial cell surface layer protein from Bacillus steatothermophilus PV72 and its application as an immobilization matrix. Appl. Microbiol. Biotechnol. 1992, 38, 147–151. [Google Scholar] [CrossRef]
- Sára, M.; Sleytr, U.B. Biotechnology and biomimetic with crystalline bacterial cell surface layers (S-layers). Micron 1996, 27, 141–156. [Google Scholar] [CrossRef]
- Sára, M.; Sleytr, U.B. Crystalline bacterial cell surface layers (S-layers): From cell structure to biomimetics. Prog. Biophys. Mol. Biol. 1996, 65, 83–111. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Sára, M. Bacterial and archaeal S-layer proteins: Structure-function relationships and their biotechnological applications. Trends Biotechnol. 1997, 15, 20–26. [Google Scholar] [CrossRef]
- Mark, S.S.; Bergkvist, M.; Yang, X.; Angert, E.R.; Batt, C.A. Self-assembly of dendrimer-encapsulated nanoparticle arrays using 2-D microbial S-layer protein biotemplates. Biomacromolecules 2006, 7, 1884–1897. [Google Scholar] [CrossRef]
- Neubauer, A.; Pum, D.; Sleytr, U.B. An Amperometric Glucose Sensor Based on Isoporous Crystalline Protein Membranes as Immobilization Matrix. Anal. Lett. 1993, 26, 1347–1360. [Google Scholar] [CrossRef]
- Neubauer, A.; Hödl, C.; Pum, D.; Sleytr, U.B. A Multistep Enzyme Sensor for Sucrose Based on S-Layer Microparticles As Immobilization Matrix. Anal. Lett. 1994, 27, 849–865. [Google Scholar] [CrossRef]
- Neubauer, A.; Pum, D.; Sleytr, U.B.; Klimant, I.; Wolfbeis, O.S. Fibre-optic glucose biosensor using enzyme membranes with 2-D crystalline structure. Biosens. Bioelectron. 1996, 11, 317–325. [Google Scholar] [CrossRef]
- Raff, J.; Matys, S.; Suhr, M.; Vogel, M.; Günther, T.; Pollmann, K. S-Layer-Based Nanocomposites for Industrial Applications. In Protein-Based Engineered Nanostructures. Advances in Experimental Medicine and Biology; Cortajarena, A.L., Grove, T.Z., Eds.; Springer: New York, NY, USA, 2016; Volume 940, pp. 245–279. [Google Scholar] [CrossRef]
- Picher, M.M.; Küpcü, S.; Huang, C.-J.; Dostalek, J.; Pum, D.; Sleytr, U.B.; Ertl, P. Nanobiotechnology advanced antifouling surfaces for the continuous electrochemical monitoring of glucose in whole blood using a lab-on-a-chip. Lab Chip 2013, 13, 1780–1789. [Google Scholar] [CrossRef]
- Pum, D.; Neubauer, A.; Sleytr, U.B.; Pentzien, S.; Reetz, S.; Kautek, W. Physico-chemical properties of crystalline nanoscale enzyme-protein-metal layer composites in biosensors. Ber. Bunsenges. Phys. Chem. 1997, 101, 1686–1689. [Google Scholar] [CrossRef]
- Neubauer, A.; Pentzien, S.; Reetz, S.; Kautek, W.; Pum, D.; Sleytr, U.B. Pulsed-laser metal contacting of biosensors on the basis of crystalline enzyme-protein layer composites. Sens. Actuators B Chem. 1997, 40, 231–236. [Google Scholar] [CrossRef]
- Ferner-Ortner-Bleckmann, J.; Schrems, A.; Ilk, N.; Egelseer, E.M.; Sleytr, U.B.; Schuster, B. Multitechnique study on a recombinantly produced Bacillus haloduranslaccase and an S-layer/laccase fusion protein. Biointerphases 2011, 6, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Küpcü, S.; Sára, M.; Sleytr, U.B. Liposomes coated with crystalline bacterial cell surface protein (S-layer) as immobilization structures for macromolecules. Biochim. Biophys. Acta Biomembr. 1995, 1235, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Ilk, N.; Egelseer, E.M.; Sleytr, U.B. S-layer fusion proteins—construction principles and applications. Curr. Opin. Biotechnol. 2011, 22, 824–831. [Google Scholar] [CrossRef] [Green Version]
- Sleytr, U.B.; Egelseer, E.M.; Ilk, N.; Pum, D.; Schuster, B. S-Layers as a basic building block in a molecular construction kit. FEBS J. 2007, 274, 323–334. [Google Scholar] [CrossRef]
- Breitwieser, A.; Küpcü, S.; Howorka, S.; Weigert, S.; Langer, C.; Hoffmann-Sommergruber, K.; Scheiner, O.; Sleytr, U.B.; Sára, M. 2-D Protein Crystals as an Immobilization Matrix for Producing Reaction Zones in Dipstick-Style Immunoassays. BioTechniques 1996, 21, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Breitwieser, A.; Mader, C.; Schocher, I.; Hoffmann-Sommergruber, K.; Aberer, W.; Scheiner, O.; Sleytr, U.B.; Sára, M. A novel dipstick developed for rapid Bet v 1-specific IgE detection: Recombinant allergen immobilized via a monoclonal antibody to crystalline bacterial cell-surface layers. Allergy 2008, 53, 786–793. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Pum, D.; Sára, M.; Schuster, B. Molecular nanotechnology with 2-D protein crystals. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; Academic Press: San Diego, CA, USA, 2004; Volume 5, pp. 693–702. [Google Scholar]
- Völkel, D.; Zimmermann, K.; Breitwieser, A.; Pable, S.; Glatzel, M.; Scheiflinger, F.; Schwarz, H.P.; Sara, M.; Sleytr, U.B.; Dorner, F. Immunochemical detection of prion protein on dipsticks prepared with crystalline bacterial cell-surface layers. Transfusion 2003, 43, 1677–1682. [Google Scholar] [CrossRef]
- Schuster, B. S-Layer Protein-Based Biosensors. Biosensors 2018, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Damiati, S.; Schuster, B. Electrochemical Biosensors Based on S-Layer Proteins. Sensors 2020, 20, 1721. [Google Scholar] [CrossRef] [Green Version]
- Hanke, W.; Schlue, W.R. Biological Techniques Series, Planar Lipid Bilayers; Academic Press: London, UK, 1993; ISBN 9780123229953. [Google Scholar] [CrossRef]
- Schuster, B.; Pum, D.; Sára, M.; Braha, O.; Bayley, H.; Sleytr, U.B. S layer ultrafiltration membranes: A new support for stabi-lizing functionalized lipid membranes. Langmuir 2001, 17, 499–503. [Google Scholar] [CrossRef]
- Schuster, B.; Weigert, S.; Pum, D.; Sára, M.; Sleytr, U.B. New Method for Generating Tetraether Lipid Membranes on Porous Supports. Langmuir 2003, 19, 2392–2397. [Google Scholar] [CrossRef]
- Gufler, P.C.; Pum, D.; Sleytr, U.B.; Schuster, B. Highly robust lipid membranes on crystalline S-layer supports investigated by electrochemical impedance spectroscopy. Biochim. Biophys. Acta Biomembr. 2004, 1661, 154–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, B.; Sleytr, U.B. Biomimetic interfaces based on S-layer proteins, lipid membranes and functional biomolecules. J. R. Soc. Interface 2014, 11, 20140232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, B. Biomimetic Design of Nanopatterned Membranes. NanoBiotechnology 2005, 1, 153–164. [Google Scholar] [CrossRef]
- Heckmann, K.; Strobl, C.; Bauer, S. Hyperfiltration through cross-linked monolayers. Thin Solid Films 1983, 99, 265–269. [Google Scholar] [CrossRef]
- Bauer, S.; Heckmann, K.; Six, L.; Strobl, C.; Blöcher, D.; Henkel, B.; Garbe, T.; Ring, K. Hyperfiltration through crosslinked monolayers II. Desalination 1983, 46, 369–378. [Google Scholar] [CrossRef]
- Schuster, B.; Sleytr, U.B. Relevance of glycosylation of S-layer proteins for cell surface properties. Acta Biomater. 2015, 19, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Tschiggerl, H.; Casey, J.L.; Parisi, K.; Foley, M.; Sleytr, U.B. Display of a Peptide Mimotope on a Crystalline Bacterial Cell Surface Layer (S-layer) Lattice for Diagnosis of Epstein–Barr Virus Infection. Bioconjug. Chem. 2008, 19, 860–865. [Google Scholar] [CrossRef]
- Breitwieser, A.; Pum, D.; Toca-Herrera, J.L.; Sleytr, U.B. Magnetic beads functionalized with recombinant S-layer protein exhibit high human IgG-binding and anti-fouling properties. Curr. Top. Pept. Protein Res. 2016, 17, 45–55. [Google Scholar]
- Genzer, J.; Marmur, A. Biological and Synthetic Self-Cleaning Surfaces. MRS Bull. 2008, 33, 742–746. [Google Scholar] [CrossRef] [Green Version]
- König, H. Archaeobacterial cell envelopes. Can. J. Microbiol. 1988, 34, 395–406. [Google Scholar] [CrossRef]
- Pfeifer, K.; Ergal, İ.; Koller, M.; Basen, M.; Schuster, B.; Rittmann, S.K.R. Archaea Biotechnology. Biotechnol. Adv. 2020, 47, 107668. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuster, B.; Sleytr, U.B. S-Layer Ultrafiltration Membranes. Membranes 2021, 11, 275. https://doi.org/10.3390/membranes11040275
Schuster B, Sleytr UB. S-Layer Ultrafiltration Membranes. Membranes. 2021; 11(4):275. https://doi.org/10.3390/membranes11040275
Chicago/Turabian StyleSchuster, Bernhard, and Uwe B. Sleytr. 2021. "S-Layer Ultrafiltration Membranes" Membranes 11, no. 4: 275. https://doi.org/10.3390/membranes11040275