2,6-Bis((benzoyl-R)amino)pyridine (R = H, 4-Me, and 4-NMe2) Derivatives for the Removal of Cu(II), Ni(II), Co(II), and Zn(II) Ions from Aqueous Solutions in Classic Solvent Extraction and a Membrane Extraction
Abstract
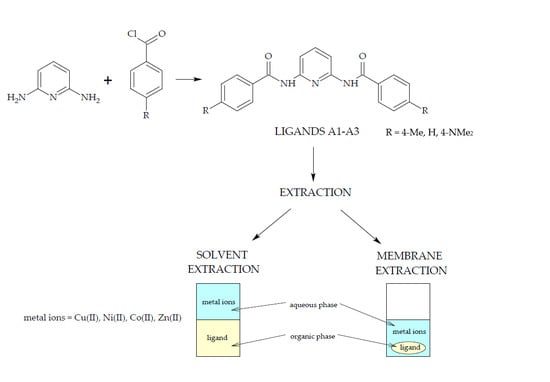
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 2,6-Bis((benzoyl-R)amino)pyridine (R = H, 4-Me, and 4-NMe2) Derivatives
- I.
- 2,6-Diaminopyridine (Sigma Aldrich, Poznan, Poland) and triethylamine (Sigma-Aldrich, Poznan, Poland) solutions were magnetically stirred in dry tetrahydrofuran (THF) (Avantor, Gliwice, Poland) (at 0 °C) and then the appropriate amount of 4-R-benzoyl chloride (solution in dry THF) was added dropwise to the mixture over a 60 min period. The 4-R-benzoyl chloride derivatives utilized included benzoyl chloride and p-toluoyl chloride (Sigma Aldrich, Poznan, Poland).
- II.
- The mixture was stirred overnight. It was heated for 1 h, evaporated, and treated by a saturated water solution of NaHCO3, and stirred for 15 min.
- III.
- The obtained solids were re-crystallized twice from ethanol (Sigma Aldrich, Poznan, Poland).
- I.
- 2,6-Diaminopyridine (Sigma Aldrich, Poznan, Poland) and sodium hydride (Sigma Aldrich, Poznan, Poland) solutions were magnetically stirred under a nitrogen atmosphere for 1 h (at 25 °C).
- II.
- The mixture was heated for 1 h and then cooled to r.t. Ethyl 4-(dimethylamino) benzoate in dry tetrahydrofuran was added dropwise to the cooled mixture over a 60 min period.
- III.
- The mixture was heated to boiling point and stirred overnight.
- IV.
- An NH4Cl solution (Sigma Aldrich, Poznan, Poland) was added to the cooled mixture and was stirred to allow ammonia to evaporate.
- V.
- The mixture was evaporated under reduced pressure and a obtained solid was crystallized from ethanol (Sigma Aldrich, Poznan, Poland).
2.2. Mass Spectrometry Experiments
2.3. Complexation Properties of 2,6-Bis(R-benzoyl-amino)pyridine (R = H, 4-Me, and 4-NMe2) Derivatives
2.4. Separation Procedure
2.4.1. Classic Solvent Extraction
2.4.2. The Membrane Extraction Process
The Preparation of Polymer Inclusion Membrane
Membrane Extraction Experiments
3. Results and discussion
3.1. Confirmation of the Structures of 2,6-Bis((benzoyl-R)amino)pyridine (R = H, 4-Me, and 4-NMe2) Derivatives Using HRMS and HCD MS/MS Methods
3.2. Complexation Properties of 2,6-Bis((benzoyl-R)amino)pyridine (R = H, 4-Me, and 4-NMe2) Derivatives
3.3. Results of Separation Processes
3.3.1. Classic Solvent Extraction
3.3.2. Membrane Extraction Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Xu, L.; Wong, W.-Y. Energy materials based on metal Schiff base complexes. Coord. Chem. Rev. 2018, 355, 180–198. [Google Scholar] [CrossRef]
- Kumar, S.; Dhar, D.N.; Saxena, P.N. Applications of metal complexes of Schiff bases—A review. J. Sci. Ind. Res. 2009, 68, 181–187. [Google Scholar]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Xue, M.; Minghua, L.; Bai, Y.; Guo, Y.; Zhang, Z. Metal ion mediation of interfacial chiral supramolecular formation of amphiphilic Schff base studied by in situ second harmonic generation. J. Phys. Chem. B 2020, 124, 8179–8187. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Tzimopoulos, D.I.; Holynska, M.; Perlepes, S.P. Oligonuclear Actinoid Complexes with Schiff Bases as Ligands-Older Achievements and Recent Progress. Int. J. Mol. Sci. 2020, 21, 555. [Google Scholar] [CrossRef]
- Dolaz, M.; Kose, M. The metal complexes of new Schiff bases containing phosphonate groups and catalytic properties for alkane oxidation. Appl. Organomet. Chem. 2019, 33, e4970. [Google Scholar] [CrossRef]
- Turan, N. Synthesis, spectroscopy, optical characteristics and parameters of Co(II), Pd(II) complexes and Schiff base ligand. J. Electron. Mater. 2019, 48, 7366–7371. [Google Scholar] [CrossRef]
- Öğretir, C.; Dal, H.; Berber, H.; Taktak, F.F. Spectroscopic determination of acid dissociation constants of some pyridyl Shiff Bases. J. Chem. Eng. Data 2006, 51, 46–50. [Google Scholar] [CrossRef]
- Fabbrizzi, L. Beauty in Chemistry: Making Artistic Molecules with Schiff Bases. J. Org. Chem. 2020, 85, 12212–12226. [Google Scholar] [CrossRef] [PubMed]
- Reffas, H.; Benabdallah, T.; Youcef, M.H.; Ilikti, H. Study on the cloud point extraction of copper(II) from an aqueous sulfate medium with N,N′-bis(salicylideneaminoethyl)amine polydentate Schiff Base into a nonionic surfactant phase. J. Chem. Eng. Data 2010, 55, 912–918. [Google Scholar] [CrossRef]
- Witt, K.; Bożejewicz, D.; Kaczorowska, M.A. N,N′-bis(salicylidene)ethylenediamine (Salen) as an active compound for the recovery of Ni(II), Cu(II), and Zn(II) ions from aqueous solution. Membranes 2020, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Campo-Cobo, L.F.; Pérez-Urbano, M.L.; Gutiérrez-Valencia, T.M.; Hoyos-Saavedra, O.L.; Cuervo-Ochoa, G. Selective Extraction of Gold with Polymeric Inclusion Membranes Based on Salen Ligands with Electron-Accepting Substituents. J. Inorg. Organomet. Polym. 2021, 1–11. [Google Scholar] [CrossRef]
- Oshima, S.; Hirayama, N.; Kubono, K.; Kokusen, H.; Honjo, T. Structural control of Schiff Base ligands for selective extraction of copper(II). Anal. Sci. 2003, 18, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Dede, B.; Karipcin, F.; Cengiz, M. Novel homo- and hetero-nuclear copper(II) complexes of tetradentate Schiff bases: Synthesis, characterization, solvent-extraction and catalase-like activity studies. J. Hazard. Mater. 2009, 163, 1148–1156. [Google Scholar] [CrossRef]
- Wiecka, Z.; Rzelewska-Piekut, M.; Wojciechowska, I.; Wieszczycka, K.; Regel-Rosocka, M. Recovery of Palladium(II) and Platinum(IV) in Novel Extraction Systems. Materials 2021, 14, 285. [Google Scholar] [CrossRef]
- Bhargava, S.; Uma, V. Rapid extraction of Cu(II) heavy metal from industraial waste water by using silver nanoparticles anchored with novel Schiff base. Sep. Sci. Technol. 2018, 54, 1181–1193. [Google Scholar] [CrossRef]
- Cheira, M.F.; Orabi, A.S.; Atia, B.M.; Hassan, S.M. Solvent extraction and separation of thorium(IV) from chloride media by a Schiff base. J. Solution Chem. 2018, 47, 611–633. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Sheikhshoaie, I. Mercury(II) ion-selective polymeric membrane sensor based on recently synthesized Schiff base. Talanta 2003, 60, 73–80. [Google Scholar] [CrossRef]
- Ulewicz, M.; Lesińska, U.; Bocheńska, M. Transport of lead across polymer inclusion membrane with p-tert-butylcalix[4]aren derivative. Physicochem. Probl. Miner. 2010, 44, 245–256. [Google Scholar]
- Ulewicz, M.; Lesinska, U.; Bocheńska, M.; Walkowiak, W. Facilitated transport of Zn(II), Cd(II) and Pb(II) ions through polymer inclusion membranes with calix[4]-crown-6 derivatives. Sep. Purif. Technol. 2007, 54, 299–305. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of Hydrophobic Alkylimidazoles in the Separation of Non-Ferrous Metal Ions across Plasticised Membranes—A Review. Membranes 2020, 10, 331. [Google Scholar] [CrossRef]
- Gajda, B.; Plackowski, R.; Skrzypczak, A.; Bogacki, M.B. Facilitated Transport of Copper(II) across Polymer Inclusion Membrane with Triazole Derivatives as Carrier. Membranes 2020, 10, 201. [Google Scholar] [CrossRef]
- Bożejewicz, D.; Witt, K.; Kaczorowska, M.A. The comparison of the removal of copper(II) and zinc(II) ions from aqueous solution using 2,6-diaminopyridine in a polymer inclusion membrane and in a classic solvent extraction. Desalin. Water Treat. 2021, 214, 194–202. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, Y.; Zhang, J.; Lin, S.; Zhang, K.; Tian, H.; Dong, Y.; Xu, H. Identification of Novel Thiazolo[5,4-b]Pyridine Derivatives as Potent Phosphoinositide 3-Kinase Inhibitors. Molecules 2020, 25, 20–4630. [Google Scholar] [CrossRef]
- Sun, J.W.; Liu, X.Y.; Guo, J.W.; Zhao, W.G.; Gao, W.P. Pyridine-2,6-dicarboxaldehyde-Enabled N-Terminal in situ Growth of Polymer-Interferon alpha Conjugates with Significantly Improved Pharmacokinetics and in vivo Bioactivity. ACS Appl. Mater. Interfaces 2021, 13, 88–96. [Google Scholar] [CrossRef]
- Alghanmi, R.M.; Habeeb, M.M. Spectral and solvation effect studies on charge transfer complex of 2,6-diaminopyridine with chloranilic acid. J. Mol. Liq. 2013, 181, 20–28. [Google Scholar] [CrossRef]
- Milošević, M.D.; Marinković, A.D.; Petrović, P.; Klaus, A.; Nikolić, M.G.; Prlainović, N.Ž.; Cvijetić, I.N. Synthesis, characterization and SAR studies of bis(imino)pyridines as antioxidants, acetylcholinesterase inhibitors and antimicrobial agents. Bioorg. Med. Chem. 2020, 102, 104073. [Google Scholar] [CrossRef]
- Mishra, A.; Bhajiwala, H.; Kothari, A.; Gupta, V.K. A new class of pyridine-amide containing Ti and Zr based catalysts for olefin polymerization: Influence of ligand substituents. Catal. Lett. 2019, 149, 3425–3431. [Google Scholar] [CrossRef]
- Chen, X.-H.; Zeng, Z.-X.; Xue, W.-L.; Pu, T. Solubility of 2,6-diaminopyridine in toluene, o-xylene, ethylbenzene, methanol, ethanol, 2-propanol and sodium hydroxide solutions. J. Chem. Eng. Data 2007, 52, 1911–1915. [Google Scholar] [CrossRef]
- Rana, V.B.; Singh, P.; Singh, D.P.; Teotia, M.P. Trivalent chromium, manganese, iron, and cobalt chelates of a tetradentate N6 macrocyclic ligand. Transit. Met. Chem. 1982, 7, 174–177. [Google Scholar] [CrossRef]
- Witt, K.; Radzymińska-Lenarcik, E.; Kościuszko, A.; Gierszewska, M.; Ziuziakowski, K. The Influence of the Morphology and Mechanical Properties of Polymer Inclusion Membranes (PIMs) on Zinc Ion Separation from Aqueous Solutions. Polymers 2018, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Konermann, L.; Ahadi, E.; Rodriguez, A.D.; Vahidi, S. Unraveling the Mechanism of Electrospray Ionization. Anal. Chem. 2013, 85, 2–9. [Google Scholar] [CrossRef]
- Shukla, A.K.; Futrell, H.J. Tandem mass spectrometry: Dissociation of ions by collisional activation. J. Mass Spectrom. 2000, 35, 1069–1090. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Huttlin, E.L.; Haas, W.; Sowa, M.E.; Rad, R.; Gygi, S.P. Evaluation of HCD- and CID-type fragmentation within their respective detection platforms for murine phosphoproteomics. Mol. Cell Proteomics. 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, M.A.; Copper, H.J. Characterization of Polyphosphoesters by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2009, 20, 2238–2247. [Google Scholar] [CrossRef]
- Newton, K.A.; McLuckey, S.A. Generation and manipulation of sodium cationized peptides in the gas phase. J. Am. Soc. Mass Spectrom. 2004, 15, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Bożejewicz, D.; Witt, K.; Kaczorowska, M.A.; Ośmiałowski, B. The copper(II) ions solvent extraction with a new compound: 2,6-bis(4-methoxybenzoyl)-diaminopyridine. Processes 2019, 7, 954. [Google Scholar] [CrossRef]
- Muthaiah, S.; Bhatia, A.; Kannan, M. Stability of Metal Complexes; Open Access Peer-Reviewed Chapter; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Cretescu, I.; Soreanu, G.; Harja, M. A low-cost sorbent for removal of copper ions from wastewaters based on sawdust/fly ash mixture. Int. J. Environ. Sci. Technol. 2015, 12, 1799–1810. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Saad, E.A.; Soliman, M.A.; Abdelwahab, M.S. Removal of radioactive cobalt/zinc and some heavy metals from water using diethylenetriamine/2-pyridinecarboxaldehyde supported on NZVI. Microchem. J. 2019, 145, 1102–1111. [Google Scholar] [CrossRef]









| Structure of compound |  |
| Chemical name | 2,6-bis(benzoylamino)pyridine (A1) |
| Molecular formula | C19H15N3O2 |
| Monoisotopic mass [Da] | 317.1164 |
| Structure of compound |  |
| Chemical name | 2,6-bis(4-methylbenzoylamino)pyridine (A2) |
| Molecular formula | C21H19N3O2 |
| Monoisotopic mass [Da] | 345.1478 |
| Structure of compound |  |
| Chemical name | 2,6-bis (4-(N,N-dimethylamino)benzoylamino)pyridine (A3) |
| Molecular formula | C23H25N5O2 |
| Monoisotopic mass [Da] | 403.2008 |
| Metal Ions | Ligand | M:L | pH | C[M] | C[L] | T | µ |
|---|---|---|---|---|---|---|---|
| Cu(II) | A1 C19H15N3O2 | 1:1 | 10.016 | 0.010 | 0.010 | 20 | 200 |
| 1:2 | 8.256 | 0.005 | 0.010 | 22 | 300 | ||
| 1:5 | 10.424 | 0.002 | 0.010 | 25 | 400 | ||
| A2 C21H19N3O2 | 1:1 | 10.145 | 0.010 | 0.010 | 20 | 200 | |
| 1:2 | 7.985 | 0.005 | 0.010 | 22 | 300 | ||
| 1:5 | 10.753 | 0.002 | 0.010 | 25 | 400 | ||
| A3 C23H25N5O2 | 1:1 | 10.160 | 0.010 | 0.010 | 20 | 200 | |
| 1:2 | 8.157 | 0.005 | 0.010 | 22 | 300 | ||
| 1:5 | 10.792 | 0.002 | 0.010 | 25 | 400 | ||
| Ni(II) | A1 C19H15N3O2 | 1:1 | 10.298 | 0.010 | 0.010 | 20 | 200 |
| 1:2 | 7.845 | 0.005 | 0.010 | 22 | 300 | ||
| 1:5 | 10.510 | 0.002 | 0.010 | 25 | 400 | ||
| A2 C21H19N3O2 | 1:1 | 10.043 | 0.010 | 0.010 | 20 | 200 | |
| 1:2 | 7.955 | 0.005 | 0.010 | 22 | 300 | ||
| 1:5 | 10.573 | 0.002 | 0.010 | 25 | 400 | ||
| A3 C23H25N5O2 | 1:1 | 10.134 | 0.010 | 0.010 | 20 | 200 | |
| 1:2 | 8.254 | 0.005 | 0.010 | 22 | 300 | ||
| 1:5 | 10.580 | 0.002 | 0.010 | 25 | 400 | ||
| Co(II) | A1 C19H15N3O2 | 1:1 | 9.920 | 0.010 | 0.010 | 20 | 200 |
| 1:2 | 8.5668 | 0.005 | 0.01 | 22 | 300 | ||
| 1:5 | 10.463 | 0.002 | 0.01 | 25 | 400 | ||
| A2 C21H19N3O2 | 1:1 | 10.050 | 0.010 | 0.01 | 20 | 200 | |
| 1:2 | 7.756 | 0.005 | 0.01 | 22 | 300 | ||
| 1:5 | 10.553 | 0.002 | 0.01 | 25 | 400 | ||
| A3 C23H25N5O2 | 1:1 | 10.139 | 0.010 | 0.01 | 20 | 200 | |
| 1:2 | 8.651 | 0.005 | 0.01 | 22 | 300 | ||
| 1:5 | 10.645 | 0.002 | 0.01 | 25 | 400 | ||
| Zn(II) | A1 C19H15N3O2 | 1:1 | 10.216 | 0.010 | 0.01 | 20 | 200 |
| 1:2 | 8.654 | 0.005 | 0.01 | 22 | 300 | ||
| 1:5 | 10.728 | 0.002 | 0.01 | 25 | 400 | ||
| A2 C21H19N3O2 | 1:1 | 10.138 | 0.010 | 0.01 | 20 | 200 | |
| 1:2 | 7.859 | 0.005 | 0.01 | 22 | 300 | ||
| 1:5 | 10.716 | 0.002 | 0.01 | 25 | 400 | ||
| A3 C23H25N5O2 | 1:1 | 10.209 | 0.010 | 0.01 | 20 | 200 | |
| 1:2 | 7.6591 | 0.005 | 0.01 | 22 | 300 | ||
| 1:5 | 10.682 | 0.002 | 0.01 | 25 | 400 |
| Metal Ions | Ligand | M:L | pH |
|---|---|---|---|
| Cu(II) | A1 C19H15N3O2 | 1:5 | 9.262 |
| A2 C21H19N3O2 | 1:5 | 9.194 | |
| A3 C23H25N5O2 | 1:5 | 9.428 | |
| Ni(II) | A1 C19H15N3O2 | 1:5 | 10.609 |
| A2 C21H19N3O2 | 1:5 | 9.332 | |
| A3 C23H25N5O2 | 1:5 | 10.895 | |
| Co(II) | A1 C19H15N3O2 | 1:5 | 10.654 |
| A2 C21H19N3O2 | 1:5 | 10.456 | |
| A3 C23H25N5O2 | 1:5 | 9.850 | |
| Zn(II) | A1 C19H15N3O2 | 1:5 | 9.345 |
| A2 C21H19N3O2 | 1:5 | 10.850 | |
| A3 C23H25N5O2 | 1:5 | 9.506 |
| Compound A1 (C19H15N3O2) | ||
|---|---|---|
| m/zmeas | m/zcalc | Assignment |
| 318.1233 | 318.1242 | [A1+H]+ |
| 340.1052 | 340.1062 | [A1+Na]+ |
| 657.2217 | 657.2226 | [2A1+Na]+ |
| Compound A2 (C21H19N3O2) | ||
| m/zmeas | m/zcalc | Assignment |
| 346.1538 | 346.1555 | [A2+H]+ |
| 368.1365 | 368.1375 | [A2+Na]+ |
| 691.3022 | 691.3032 | [2A2+H]+ |
| 713.2841 | 713.2852 | [2A2+Na]+ |
| Compound A3 (C23H25N5O2) | ||
| m/zmeas | m/zcalc | Assignment |
| 257.1393 | 257.1402 | [C14H17N4O]+ |
| 404.2077 | 404.2086 | [A3+H]+ |
| 426.1896 | 426.1906 | [A3+Na]+ |
| 660.3402 | 660.3410 | [A3+(C14H17N4O)]+ |
| [A1+H]+ | ESI HCD MS/MS mass spectrum of [A1+H]+ | ||
|---|---|---|---|
| m/zmeas | m/zcalc | Assignment |  |
| 318.1235 | 318.1242 | [A1+H]+, (C19H16N3O2) | |
| 300.1129 | 300.1137 | [A1-H2O+H]+, (C19H14N3O1) | |
| 282.1032 | 282.1031 | [A1-2H2O+H]+, (C19H12N3) | |
| 215.0815 | 215.0820 | [C12H11N2O2]+ | |
| 197.0709 | 197.0715 | [C12H9N2O]+ | |
| 171.0553 | 171.0558 | [C10H7N2O]+ | |
| 105.0339 | 105.0340 | [C7H5O]+ | |
| 95.0496 | 95.0497 | [C6H7O]+ | |
| 77.0392 | 77.0391 | [C6H5]+ | |
| [A2+H]+ | ESI HCD MS/MS mass spectrum of [A2+H]+ | ||
| m/zmeas | m/zcalc | Assignment |  |
| 346.1538 | 346.1555 | [A2+H]+, (C21H20N3O2) | |
| 328.1434 | 328.1450 | [A2-H2O+H]+, (C21H18N3O1) | |
| 310.1330 | 310.1344 | [A2-2H2O+H]+, (C21H16N3) | |
| 254.0917 | 254.0929 | [C14H12N3O2]+ | |
| 229.0965 | 229.0977 | [C13H13N2O2]+ | |
| 211.0860 | 211.0871 | [C13H11N2O]+ | |
| 185.0704 | 185.0715 | [C11H9N2O]+ | |
| 119.0490 | 119.0497 | [C8H7O]+ | |
| 109.0649 | 109.0653 | [C7H9O]+ | |
| 91.0545 | 91.0548 | [C7H7]+ | |
| [A3+H]+ | ESI HCD MS/MS mass spectrum of [A3+H]+ | ||
| m/zmeas | m/zcalc | Assignment |  |
| 404.2068 | 404.2086 | [A3+H]+, (C23H26N5O2) | |
| 386.1963 | 386.1981 | [A3-H2O+H]+, (C23H24N5O1) | |
| 356.1463 | 356.1511 | [A3-H2O-C2H6+H]+, (C21H18N5O1) | |
| 283.1180 | 283.1195 | [C15H15N4O2]+ | |
| 214.0967 | 214.0980 | [C12H12N3O]+ | |
| 148.0751 | 148.0762 | [C9H10NO]+ | |
| 122.0963 | 122.0970 | [C8H12N]+ | |
| 79.0547 | 79.0548 | [C6H7]+ | |
| Metal Ions | A1—C19H15N3O2M = 317.34 g/mol | A2—C21H19N3O2M = 345.39 g/mol | A3—C23H25N5O2M = 403.47 g/mol | |||
|---|---|---|---|---|---|---|
| log K1 | log K2 | log K1 | log K2 | log K1 | log K2 | |
| Cu(II) | 4.398 | 3.475 | 4.225 | 4.724 | 8.266 | 9.370 |
| Ni(II) | 4.544 | 4.515 | 4.500 | 4.605 | 7.695 | 8.131 |
| Co(II) | 4.183 | 4.583 | 5.809 | 6.415 | 4.218 | 5.836 |
| Zn(II) | 3.848 | 4.942 | 5.377 | 5.870 | 5.908 | 6.164 |
| A1 | A2 | A3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Metal Ions | Cm [mol/dm3] | DM | Metal Ions | Cm [mol/dm3] | DM | Metal Ions | Cm [mol/dm3] | DM |
| Cu(II) | 0.01 | 0.152 | Cu(II) | 0.01 | 0.557 | Cu(II) | 0.01 | 0.141 |
| 0.002 | 3.939 | 0.002 | 3.689 | 0.002 | 3.398 | |||
| 0.005 | 0.970 | 0.005 | 1.135 | 0.005 | 0.785 | |||
| Ni(II) | 0.01 | 21.486 | Ni(II) | 0.01 | 42.765 | Ni(II) | 0.01 | 4.944 |
| 0.002 | 4.953 | 0.002 | 137.861 | 0.002 | 17.993 | |||
| 0.005 | 3.921 | 0.005 | 11.722 | 0.005 | 8.652 | |||
| Co(II) | 0.01 | 343.710 | Co(II) | 0.01 | 144.544 | Co(II) | 0.01 | 88.719 |
| 0.002 | 281.621 | 0.002 | 731.722 | 0.002 | 164.552 | |||
| 0.005 | 67.493 | 0.005 | 73.529 | 0.005 | 28.850 | |||
| Zn(II) | 0.01 | 4.327 | Zn(II) | 0.01 | 7.056 | Zn(II) | 0.01 | 4.333 |
| 0.002 | 4.057 | 0.002 | 3.653 | 0.002 | 4.256 | |||
| 0.005 | 4.035 | 0.005 | 4.461 | 0.005 | 4.274 | |||
| Compound | Metal Ions | qt |
|---|---|---|
| A1 | Cu(II) | 0.17 |
| Ni(II) | 0.30 | |
| Co(II) | 3.42 | |
| Zn(II) | 2.44 | |
| A2 | Cu(II) | 0.79 |
| Ni(II) | 0.92 | |
| Co(II) | 2.12 | |
| Zn(II) | 3.69 | |
| A3 | Cu(II) | 0.21 |
| Ni(II) | 0.60 | |
| Co(II) | 2.07 | |
| Zn(II) | 3.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bożejewicz, D.; Ośmiałowski, B.; Kaczorowska, M.A.; Witt, K. 2,6-Bis((benzoyl-R)amino)pyridine (R = H, 4-Me, and 4-NMe2) Derivatives for the Removal of Cu(II), Ni(II), Co(II), and Zn(II) Ions from Aqueous Solutions in Classic Solvent Extraction and a Membrane Extraction. Membranes 2021, 11, 233. https://doi.org/10.3390/membranes11040233
Bożejewicz D, Ośmiałowski B, Kaczorowska MA, Witt K. 2,6-Bis((benzoyl-R)amino)pyridine (R = H, 4-Me, and 4-NMe2) Derivatives for the Removal of Cu(II), Ni(II), Co(II), and Zn(II) Ions from Aqueous Solutions in Classic Solvent Extraction and a Membrane Extraction. Membranes. 2021; 11(4):233. https://doi.org/10.3390/membranes11040233
Chicago/Turabian StyleBożejewicz, Daria, Borys Ośmiałowski, Małgorzata Anna Kaczorowska, and Katarzyna Witt. 2021. "2,6-Bis((benzoyl-R)amino)pyridine (R = H, 4-Me, and 4-NMe2) Derivatives for the Removal of Cu(II), Ni(II), Co(II), and Zn(II) Ions from Aqueous Solutions in Classic Solvent Extraction and a Membrane Extraction" Membranes 11, no. 4: 233. https://doi.org/10.3390/membranes11040233