Recent Progress in One- and Two-Dimensional Nanomaterial-Based Electro-Responsive Membranes: Versatile and Smart Applications from Fouling Mitigation to Tuning Mass Transport
Abstract
:1. Introduction
2. Nanomaterials for Electro-Responsive Membranes
3. Preparation of Electro-Responsive Membranes
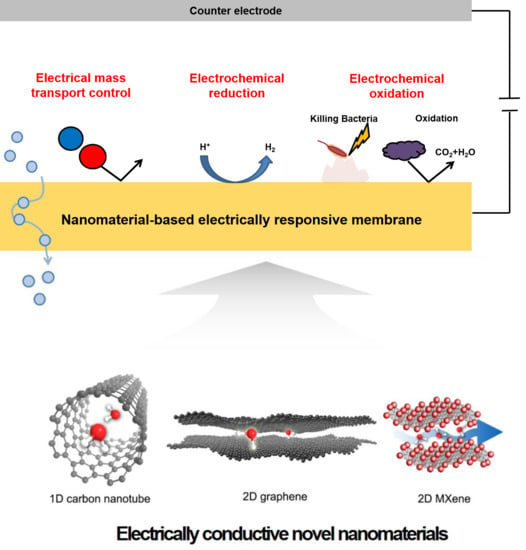
4. Versatility and Working Principle of Electro-Responsive Membranes
5. Applications of Electrically Responsive Membranes
5.1. Fouling Mitigation
5.2. Self-Cleaning
5.3. Fouling Monitoring
5.4. Organic Contaminant Removal by Electrochemical Oxidation
5.5. Controlling Water Permeability
5.6. Enhancing Ion and Organic Dye Molecule Rejections
5.7. Wettability Mitigation in the Membrane Distillation Process
5.8. Energy Recovery in A Bioelectrochemical System
6. Current Challenges and Outlook
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gude, V.G. Desalination and water reuse to address global water scarcity. Rev. Env. Sci. BioTechnol. 2017, 16, 591–609. [Google Scholar] [CrossRef]
- Bennett, A. Developments in desalination and water reuse. Filtr. Sep. 2015, 52, 28–33. [Google Scholar] [CrossRef]
- Ridgway, H.F.; Orbell, J.; Gray, S. Molecular simulations of polyamide membrane materials used in desalination and water reuse applications: Recent developments and future prospects. J. Membr. Sci. 2017, 524, 436–448. [Google Scholar] [CrossRef]
- Yang, J.; Monnot, M.; Ercolei, L.; Moulin, P. Membrane-based processes used in municipal wastewater treatment for water reuse: State-of-the-art and performance analysis. Membranes 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Sodiq, A.; Giwa, A.; Eke, J.; Pikuda, O.; De Luca, G.; Di Salvo, J.L.; Chakraborty, S. A review of emerging trends in membrane science and technology for sustainable water treatment. J. Clean. Prod. 2020, 266, 121867. [Google Scholar] [CrossRef]
- Suwaileh, W.; Johnson, D.; Hilal, N. Membrane desalination and water re-use for agriculture: State of the art and future outlook. Desalination 2020, 491, 114559. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yildirim, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, Ş.B.; Chen, Y.; et al. MXene materials for designing advanced separation membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef]
- Goh, K.; Karahan, H.E.; Wei, L.; Bae, T.-H.; Fane, A.G.; Wang, R.; Chen, Y. Carbon nanomaterials for advancing separation membranes: A strategic perspective. Carbon 2016, 109, 694–710. [Google Scholar] [CrossRef]
- Ying, Y.; Ying, W.; Li, Q.; Meng, D.; Ren, G.; Yan, R.; Peng, X. Recent advances of nanomaterial-based membrane for water purification. Appl. Martar. Today 2017, 7, 144–158. [Google Scholar] [CrossRef]
- Li, B.L.; Wang, J.; Zou, H.L.; Garaj, S.; Lim, C.T.; Xie, J.; Li, N.B.; Leong, D.T. Low-dimensional transition metal dichalcogenide nanostructures based sensors. Adv. Func. Matar. 2016, 26, 7034–7056. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. One-dimensional and two-dimensional synergized nanostructures for high-performing energy storage and conversion. Informat 2020, 2, 3–32. [Google Scholar] [CrossRef] [Green Version]
- Konduri, S.; Tong, H.M.; Chempath, S.; Nair, S. Water in single-walled aluminosilicate nanotubes: Diffusion and adsorption properties. J. Phys. Chem. C 2008, 112, 15367–15374. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Grossman, J.C. Water desalination across nanoporous graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef]
- Zhu, X.; Jassby, D. Electroactive membranes for water treatment: Enhanced treatment functionalities, energy considerations, and future challenges. Acc. Chem. Res. 2019, 52, 1177–1186. [Google Scholar] [CrossRef]
- Ahmed, F.; Lalia, B.S.; Kochkodan, V.; Hilal, N.; Hashaikeh, R. Electrically conductive polymeric membranes for fouling prevention and detection: A review. Desalination 2016, 391, 1–15. [Google Scholar] [CrossRef]
- Si, Y.; Sun, C.; Li, D.; Yang, F.; Tang, C.Y.; Quan, X.; Dong, Y.; Guiver, M.D. Flexible superhydrophobic metal-based carbon nanotube membrane for electrochemically enhanced water treatment. Environ. Sci. Technol. 2020, 54, 9074–9082. [Google Scholar] [CrossRef]
- Rao, U.; Iddya, A.; Jung, B.; Khor, C.M.; Hendren, Z.; Turchi, C.; Cath, T.; Hoek, E.M.; Ramon, G.Z.; Jassby, D. Mineral scale prevention on electrically conducting membrane distillation membranes using induced electrophoretic mixing. Environ. Sci. Technol. 2020, 54, 3678–3690. [Google Scholar] [CrossRef]
- Dudchenko, A.V.; Rolf, J.; Russell, K.; Duan, W.; Jassby, D. Organic fouling inhibition on electrically conducting carbon nanotube–polyvinyl alcohol composite ultrafiltration membranes. J. Membr. Sci. 2014, 468, 1–10. [Google Scholar] [CrossRef]
- Yuan, X.-S.; Guo, Z.-Y.; Geng, H.-Z.; Rhen, D.S.; Wang, L.; Yuan, X.-T.; Li, J. Enhanced performance of conductive polysulfone/MWCNT/PANI ultrafiltration membrane in an online fouling monitoring application. J. Membr. Sci. 2019, 575, 160–169. [Google Scholar] [CrossRef]
- Sun, J.; Hu, C.; Wu, B.; Qu, J. Fouling mitigation of a graphene hydrogel membrane electrode by electrical repulsion and in situ self-cleaning in an electro-membrane reactor. Chem. Eng. J. 2020, 393, 124817. [Google Scholar] [CrossRef]
- Hashaikeh, R.; Lalia, B.S.; Kochkodan, V.; Hilal, N. A novel in situ membrane cleaning method using periodic electrolysis. J. Membr. Sci. 2014, 471, 149–154. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Zhao, C.; Liu, J.; Yang, F. Hydraulic power and electric field combined antifouling effect of a novel conductive poly (aminoanthraquinone)/reduced graphene oxide nanohybrid blended PVDF ultrafiltration membrane. J. Mater. Chem. A 2015, 3, 20277–20287. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, X.; Fan, X.; Yi, G.; Chen, S.; Yu, H.; Chen, Y. Improving ion rejection of conductive nanofiltration membrane through electrically enhanced surface charge density. Environ. Sci. Technol. 2018, 53, 868–877. [Google Scholar] [CrossRef]
- Chen, J.; Leng, L.; Ye, C.; Lu, Q.; Addy, M.; Wang, J.; Liu, J.; Chen, P.; Ruan, R.; Zhou, W. A comparative study between fungal pellet- and spore-assisted microalgae harvesting methods for algae bioflocculation. Bioresour. Technol. 2018, 259, 181–190. [Google Scholar] [CrossRef]
- Ren, C.E.; Alhabeb, M.; Byles, B.W.; Zhao, M.-Q.; Anasori, B.; Pomerantseva, E.; Mahmoud, K.A.; Gogotsi, Y. Voltage-gated ions sieving through 2D MXene Ti3C2T x membranes. ACS Appl. Nano Mater. 2018, 1, 3644–3652. [Google Scholar] [CrossRef]
- Malaeb, L.; Katuri, K.P.; Logan, B.E.; Maab, H.; Nunes, S.P.; Saikaly, P.E. A hybrid microbial fuel cell membrane bioreactor with a conductive ultrafiltration membrane biocathode for wastewater treatment. Environ. Sci. Technol. 2013, 47, 11821–11828. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Wang, Z.; Liu, L.; Liu, H.; Wang, J. Electrothermally driven membrane distillation for low-energy consumption and wetting mitigation. Environ. Sci. Technol. 2019, 53, 13506–13513. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, X.; Wang, X.; Fan, S.; Zhou, Q.; Shao, H.; Hu, W.; Li, C.; Tong, L.; Kumar, R.R.; et al. Membrane fouling mitigation by coupling applied electric field in membrane system: Configuration, mechanism and performance. Electrochim. Acta 2018, 287, 124–134. [Google Scholar] [CrossRef]
- Trellu, C.; Chaplin, B.P.; Coetsier, C.; Esmilaire, R.; Cerneaux, S.; Causserand, C.; Cretin, M. Electro-oxidation of organic pollutants by reactive electrochemical membranes. Chemosphere 2018, 208, 159–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formoso, P.; Pantuso, E.; De Filpo, G.; Nicoletta, F.P. Electro-conductive membranes for permeation enhancement and fouling mitigation: A short review. Membranes 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronen, A.; Walker, S.L.; Jassby, D. Electroconductive and electroresponsive membranes for water treatment. Rev. Chem. Eng. 2016, 32, 533–550. [Google Scholar] [CrossRef]
- Manawi, Y.; Kochkodan, V.; Hussein, M.A.; Khaleel, M.A.; Khraisheh, M.; Hilal, N. Can carbon-based nanomaterials revolutionize membrane fabrication for water treatment and desalination? Desalination 2016, 391, 69–88. [Google Scholar] [CrossRef]
- Mihut, D.M.; Afshar, A. Electrically assisted silver and copper coated filter papers with enhanced bactericidal effects. Colloids Surf. A Phys. Eng. Asp. 2020, 606, 125428. [Google Scholar] [CrossRef]
- Trellu, C.; Rivallin, M.; Cerneaux, S.; Coetsier, C.; Causserand, C.; Oturan, M.A.; Cretin, M. Integration of sub-stoichiometric titanium oxide reactive electrochemical membrane as anode in the electro-Fenton process. Chem. Eng. J. 2020, 400, 125936. [Google Scholar] [CrossRef]
- Fan, Y.; Li, J.; Wang, S.; Meng, X.; Zhang, W.; Jin, Y.; Yang, N.; Tan, X.; Li, J.; Liu, S. Voltage-enhanced ion sieving and rejection of Pb2+ through a thermally cross-linked two-dimensional MXene membrane. Chem. Eng. J. 2020, 401, 126073. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.; Rao, A. Carbon nanotubes. In The Physics of Fullerene-Based and Fullerene-Related Materials; Springer: Berlin/Heidelberg, Germany, 2000; pp. 331–379. [Google Scholar]
- Kar, S.; Bindal, R.; Tewari, P. Carbon nanotube membranes for desalination and water purification: Challenges and opportunities. Nano Today 2012, 7, 385–389. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, G.J. Electrical conductivity of carbon nanotube- and graphene-based nanocomposites. In Micromechanics and Nanomechanics of Composite Solids; Meguid, S.A., Weng, G.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 123–156. [Google Scholar]
- Santos, M.C.; Elabd, Y.A.; Jing, Y.; Chaplin, B.P.; Fang, L. Highly porous Ti4O7 reactive electrochemical water filtration membranes fabricated via electrospinning/electrospraying. ALCHE J. 2016, 62, 508–524. [Google Scholar] [CrossRef]
- Zaky, A.M.; Chaplin, B.P. Porous substoichiometric TiO2 anodes as reactive electrochemical membranes for water treatment. Envion. Sci. Technol. 2013, 47, 6554–6563. [Google Scholar] [CrossRef]
- Zaky, A.M.; Chaplin, B.P. Mechanism of p-substituted phenol oxidation at a Ti4O7 reactive electrochemical membrane. Envion. Sci. Technol. 2014, 48, 5857–5867. [Google Scholar] [CrossRef]
- Krinks, J.K.; Qiu, M.; Mergos, I.A.; Weavers, L.K.; Mouser, P.J.; Verweij, H. Piezoceramic membrane with built-in ultrasonic defouling. J. Membr. Sci. 2015, 494, 130–135. [Google Scholar] [CrossRef]
- Mi, B. Graphene oxide membranes for ionic and molecular sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Alemour, B.; Yaacob, M.; Lim, H.; Hassan, M.R. Review of electrical properties of graphene conductive composites. Int. J. Nanoelectron. Mater. 2018, 11, 371–398. [Google Scholar]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Qiu, G.; Zhou, Z.; Li, J.; Amy, G.L.; Xie, J.; Lee, J.Y. An effective design of electrically conducting thin-film composite (TFC) membranes for bio and organic fouling control in forward osmosis (FO). Environ. Sci. Technol. 2016, 50, 10596–10605. [Google Scholar] [CrossRef]
- De Lannoy, C.F.; Jassby, D.; Davis, D.D.; Wiesner, M.R. A highly electrically conductive polymer–multiwalled carbon nanotube nanocomposite membrane. J. Membr. Sci. 2012, 415–416, 718–724. [Google Scholar] [CrossRef]
- Duan, W.; Ronen, A.; Walker, S.; Jassby, D. Polyaniline-coated carbon nanotube ultrafiltration membranes: Enhanced anodic stability for in situ cleaning and electro-oxidation processes. ACS Appl. Mater. Interfaces 2016, 8, 22574–22584. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, F.; Liu, J.; Yang, F. EMBR. J. Membr. Sci. 2013, 437, 99–107. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Wang, K.; Li, K.; Xu, L.; Wang, J.; Wang, J. Fabrication of a novel conductive ultrafiltration membrane and its application for electrochemical removal of hexavalent chromium. J. Membr. Sci. 2019, 584, 191–201. [Google Scholar] [CrossRef]
- Du, L.; Quan, X.; Fan, X.; Wei, G.; Chen, S. Conductive CNT/nanofiber composite hollow fiber membranes with electrospun support layer for water purification. J. Membr. Sci. 2020, 596, 117613. [Google Scholar] [CrossRef]
- Shakeri, A.; Salehi, H.; Rastgar, M. Antifouling electrically conductive membrane for forward osmosis prepared by polyaniline/graphene nanocomposite. J. Water Process. Eng. 2019, 32, 100932. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.-H. Harnessing filler materials for enhancing biogas separation membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [CrossRef] [PubMed]
- Omi, F.R.; Choudhury, M.R.; Anwar, N.; Bakr, A.R.; Rahaman, M.S. Highly conductive ultrafiltration membrane via vacuum filtration assisted layer-by-layer deposition of functionalized carbon nanotubes. Ind. Eng. Chem. Res. 2017, 56, 8474–8484. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Quan, X. A novel reduced graphene oxide/carbon nanotube hollow fiber membrane with high forward osmosis performance. Desalination 2019, 451, 117–124. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, W.; Li, R.; Xu, Y.; Liu, Y.; Sun, T.; Shen, L.; Lin, H. Electric field endowing the conductive polyvinylidene fluoride (PVDF)-graphene oxide (GO)‑nickel (Ni) membrane with high-efficient performance for dye wastewater treatment. Appl. Surf. Sci. 2019, 483, 1006–1016. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, S.; Jin, R.; Zhou, J.; Quan, X. A novel aerobic electrochemical membrane bioreactor with CNTs hollow fiber membrane by electrochemical oxidation to improve water quality and mitigate membrane fouling. Water Res. 2019, 151, 54–63. [Google Scholar] [CrossRef]
- Jiang, W.-L.; Xia, X.; Han, J.-L.; Ding, Y.-C.; Haider, M.R.; Wang, A.-J. Graphene modified electro-fenton catalytic membrane for in situ degradation of antibiotic florfenicol. Environ. Sci. Technol. 2018, 52, 9972–9982. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Hu, C.-Y.; Lo, S.-L. Direct and indirect electrochemical oxidation of amine-containing pharmaceuticals using graphite electrodes. J. Hazard. Mater. 2019, 366, 592–605. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, K.; Wang, X.; Liang, S.; Wei, C.; Wen, X.; Huang, X.J. Outlining the roles of membrane-foulant and foulant-foulant interactions in organic fouling during microfiltration and ultrafiltration: A mini-review. Front. Chem. 2020, 8, 417. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Recent advances in hydrophilic modification and performance of polyethersulfone (PES) membrane via additive blending. RSC Adv. 2018, 8, 22710–22728. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Shi, Y.; Jegatheesan, V.; Haq, I.U. Review on the mechanism, impacts and control methods of membrane fouling in MBR system. Membranes 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, E.; Alayande, A.B.; Kim, C.-M.; Song, J.-h.; Kim, I.S. Laminar reduced graphene oxide membrane modified with silver nanoparticle-polydopamine for water/ion separation and biofouling resistance enhancement. Desalination 2018, 426, 21–31. [Google Scholar] [CrossRef]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, J.; Sun, H.; Pan, D.; Huang, Y.; Bai, Y.; Shao, L. Thermo-responsive separation membrane with smart anti-fouling and self-cleaning properties. J. Chem. Eng. Res. Des. 2020, 156, 333–342. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, L.; Wang, R.; Jia, N.; Liu, L.; Gao, C. Bioinspired synthesis of polyzwitterion/titania functionalized carbon nanotube membrane with superwetting property for efficient oil-in-water emulsion separation. J. Membr. Sci. 2019, 589, 117257. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Yan, L.; Bai, Y.; Li, S.; Sorokin, P.; Shao, L. Biomimetic nanoparticle-engineered superwettable membranes for efficient oil/water separation. J. Membr. Sci. 2021, 618, 118525. [Google Scholar] [CrossRef]
- Chen, S.; Wang, G.; Li, S.; Li, X.; Yu, H.; Quan, X. Porous carbon membrane with enhanced selectivity and antifouling capability for water treatment under electrochemical assistance. J. Colloid Interface Sci. 2020, 560, 59–68. [Google Scholar] [CrossRef]
- Ho, K.; Teow, Y.; Mohammad, A.; Ang, W.; Lee, P.H. Development of graphene oxide (GO)/multi-walled carbon nanotubes (MWCNTs) nanocomposite conductive membranes for electrically enhanced fouling mitigation. J. Membr. Sci. 2018, 552, 189–201. [Google Scholar] [CrossRef]
- Ronen, A.; Duan, W.; Wheeldon, I.; Walker, S.; Jassby, D. Microbial attachment inhibition through low-voltage electrochemical reactions on electrically conducting membranes. Environ. Sci. Technol. 2015, 49, 12741–12750. [Google Scholar] [CrossRef]
- De Lannoy, C.-F.; Jassby, D.; Gloe, K.; Gordon, A.D.; Wiesner, M.R. Aquatic biofouling prevention by electrically charged nanocomposite polymer thin film membranes. Environ. Sci. Technol. 2013, 47, 2760–2768. [Google Scholar] [CrossRef]
- Li, B.; Sun, D.; Li, B.; Tang, W.; Ren, P.; Yu, J.; Zhang, J. One-step electrochemically prepared graphene/polyaniline conductive filter membrane for permeation enhancement by fouling mitigation. Langmuir 2020, 36, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, L.; Zhu, L. Electrically conductive membranes for anti-biofouling in membrane distillation with two novel operation modes: Capacitor mode and resistor mode. Water Res. 2019, 161, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Karkooti, A.; Rastgar, M.; Nazemifard, N.; Sadrzadeh, M. Graphene-based electro-conductive anti-fouling membranes for the treatment of oil sands produced water. Sci. Total. Environ. 2020, 704, 135365. [Google Scholar] [CrossRef] [PubMed]
- Subtil, E.L.; Goncalves, J.; Lemos, H.G.; Venancio, E.C.; Mierzwa, J.C.; de Souza, J.D.; Alves, W.; Le-Clech, P. Preparation and characterization of a new composite conductive polyethersulfone membrane using polyaniline (PANI) and reduced graphene oxide (rGO). Chem. Eng. J. 2020, 124612. [Google Scholar] [CrossRef]
- Wang, X.; Sun, M.; Zhao, Y.; Wang, C.; Ma, W.; Wong, M.S.; Elimelech, M. In situ electrochemical generation of reactive chlorine species for efficient ultrafiltration membrane self-cleaning. Environ. Sci. Technol. 2020, 54, 6997–7007. [Google Scholar] [CrossRef]
- Lalia, B.S.; Ahmed, F.E.; Shah, T.; Hilal, N.; Hashaikeh, R. Electrically conductive membranes based on carbon nanostructures for self-cleaning of biofouling. Desalination 2015, 360, 8–12. [Google Scholar] [CrossRef]
- Tay, K.G.; Song, L. A more effective method for fouling characterization in a full-scale reverse osmosis process. Desalination 2005, 177, 95–107. [Google Scholar] [CrossRef]
- Yuan, X.-T.; Xu, C.-X.; Geng, H.-Z.; Ji, Q.; Wang, L.; He, B.; Jiang, Y.; Kong, J.; Li, J. Multifunctional PVDF/CNT/GO mixed matrix membranes for ultrafiltration and fouling detection. J. Hazard. Matar. 2020, 384, 120978. [Google Scholar] [CrossRef]
- Zhang, N.; Halali, M.A.; de Lannoy, C.-F. Detection of fouling on electrically conductive membranes by electrical impedance spectroscopy. Sep. Purif. Technol. 2020, 242, 116823. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hilal, N.; Hashaikeh, R. Electrically conductive membranes for in situ fouling detection in membrane distillation using impedance spectroscopy. J. Membr. Sci. 2018, 556, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lee, J.H.D.; Xia, Q.; Ma, Y.; Yu, Y.; Yung, L.Y.; Xie, J.; Ong, C.N.; Vecitis, C.D.; Zhou, Z. A graphene-based electrochemical filter for water purification. J. Matar. Chem. A 2014, 2, 16554–16562. [Google Scholar] [CrossRef]
- Mameda, N.; Park, H.-J.; Choo, K.-H. Membrane electro-oxidizer: A new hybrid membrane system with electrochemical oxidation for enhanced organics and fouling control. Water Res. 2017, 126, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.-G.; Vasu, K.; Cherian, C.; Neek-Amal, M.; Zhang, J.C.; Ghorbanfekr-Kalashami, H.; Huang, K.; Marshall, O.; Kravets, V.; Abraham, J. Electrically controlled water permeation through graphene oxide membranes. Nature 2018, 559, 236–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Qiao, S.; Zheng, M.; Zhou, J.; Quan, X. Enhanced permeability, contaminants removal and antifouling ability of CNTs-based hollow fiber membranes under electrochemical assistance. J. Membr. Sci. 2019, 582, 335–341. [Google Scholar] [CrossRef]
- Yang, E.; Chae, K.-J.; Choi, M.-J.; He, Z.; Kim, I.S. Critical review of bioelectrochemical systems integrated with membrane-based technologies for desalination, energy self-sufficiency, and high-efficiency water and wastewater treatment. Desalination 2019, 452, 40–67. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Ren, Y.; Wang, X. Preparation of conductive microfiltration membrane and its performance in a coupled configuration of membrane bioreactor with microbial fuel cell. RSC Adv. 2017, 7, 20824–20832. [Google Scholar] [CrossRef] [Green Version]
- Njuguna, J.; Pielichowski, K.; Zhu, H. Health and Environmental Safety of Nanomaterials: Polymer Nancomposites and Other Materials Containing Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alayande, A.B.; Goh, K.; Son, M.; Kim, C.-M.; Chae, K.-J.; Kang, Y.; Jang, J.; Kim, I.S.; Yang, E. Recent Progress in One- and Two-Dimensional Nanomaterial-Based Electro-Responsive Membranes: Versatile and Smart Applications from Fouling Mitigation to Tuning Mass Transport. Membranes 2021, 11, 5. https://doi.org/10.3390/membranes11010005
Alayande AB, Goh K, Son M, Kim C-M, Chae K-J, Kang Y, Jang J, Kim IS, Yang E. Recent Progress in One- and Two-Dimensional Nanomaterial-Based Electro-Responsive Membranes: Versatile and Smart Applications from Fouling Mitigation to Tuning Mass Transport. Membranes. 2021; 11(1):5. https://doi.org/10.3390/membranes11010005
Chicago/Turabian StyleAlayande, Abayomi Babatunde, Kunli Goh, Moon Son, Chang-Min Kim, Kyu-Jung Chae, Yesol Kang, Jaewon Jang, In S. Kim, and Euntae Yang. 2021. "Recent Progress in One- and Two-Dimensional Nanomaterial-Based Electro-Responsive Membranes: Versatile and Smart Applications from Fouling Mitigation to Tuning Mass Transport" Membranes 11, no. 1: 5. https://doi.org/10.3390/membranes11010005