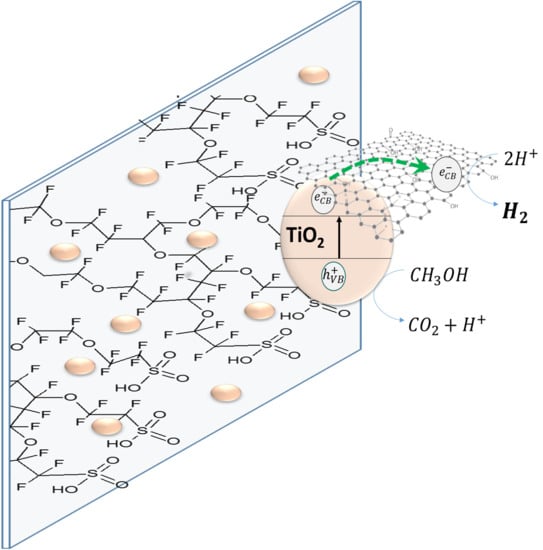
Performance of rGO/TiO2 Photocatalytic Membranes for Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Photocatalyst Preparation and Characterization
2.3. Hydrogen Production
3. Results
3.1. Materials Characterization
3.2. Photocatalytic Membrane Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Figaj, R.; Vanoli, L. Hybrid and novel solar hydrogen systems. In Solar Hydrogen Production; Calise, F., D’Accadia, M.D., Santarelli, M., Lanzini, A., Ferrero, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 487–510. ISBN 978-0-12-814853-2. [Google Scholar]
- Petkov, I.; Gabrielli, P. Power-to-hydrogen as seasonal energy storage: An uncertainty analysis for optimal design of low-carbon multi-energy systems. Appl. Energy 2020, 274, 115197. [Google Scholar] [CrossRef]
- Nolan, H.; Browne, M.P. Hydrogen energy currency: Beyond state-of-the-art transition metal oxides for oxygen electrocatalysis. Curr. Opin. Electrochem. 2020, 21, 55–61. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Albo, J.; Solla-Gullón, J.; Montiel, V.; Irabien, A. Cu oxide/ZnO-based surfaces for a selective ethylene production from gas-phase CO2 electroconversion. J. CO2 Util. 2019, 31, 135–142. [Google Scholar] [CrossRef]
- Ribao, P.; Alexandra Esteves, M.; Fernandes, V.R.; Rivero, M.J.; Rangel, C.M.; Ortiz, I. Challenges arising from the use of TiO2/rGO/Pt photocatalysts to produce hydrogen from crude glycerol compared to synthetic glycerol. Int. J. Hydrogen Energy 2018, 44, 28494–28506. [Google Scholar] [CrossRef] [Green Version]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen production: A rift into the future energy supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef] [Green Version]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Partidário, P.; Aguiar, R.; Martins, P.; Rangel, C.M.; Cabrita, I. The hydrogen roadmap in the Portuguese energy system–Developing the P2G case. Int. J. Hydrogen Energy 2019. [Google Scholar] [CrossRef] [Green Version]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef] [Green Version]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2018, 44, 540–577. [Google Scholar] [CrossRef]
- Ribao, P.; Rivero, M.J.; Ortiz, I. TiO2 structures doped with noble metals and/or graphene oxide to improve the photocatalytic degradation of dichloroacetic acid. Environ. Sci. Pollut. Res. 2017, 24, 12628–12637. [Google Scholar] [CrossRef] [PubMed]
- Beasley, C.; Kumaran Gnanamani, M.; Santillan-Jimenez, E.; Martinelli, M.; Shafer, W.D.; Hopps, S.D.; Wanninayake, N.; Kim, D.Y. Effect of Metal Work Function on Hydrogen Production from Photocatalytic Water Splitting with MTiO2 Catalysts. ChemistrySelect 2020, 5, 1013–1019. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Che, Y.; Liu, Q.; Lu, B.; Zhai, J.; Wang, K.; Liu, Z. Plasmonic ternary hybrid photocatalyst based on polymeric g-C3N4 towards visible light hydrogen generation. Sci. Rep. 2020, 10, 721. [Google Scholar] [CrossRef] [Green Version]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Ribao, P.; Rivero, M.J.; Ortiz, I. Enhanced photocatalytic activity using GO/TiO2 catalyst for the removal of DCA solutions. Environ. Sci. Pollut. Res. 2018, 25, 34893–34902. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Bahadur, N.; Dumée, L.F. Photo-catalytic membrane reactors for the remediation of persistent organic pollutants–A review. Sep. Purif. Technol. 2020, 230, 115878. [Google Scholar] [CrossRef]
- Romay, M.; Diban, N.; Rivero, M.J.; Urtiaga, A.; Ortiz, I. Critical Issues and Guidelines to Improve the Performance of Photocatalytic Polymeric Membranes. Catalysts 2020, 10, 570. [Google Scholar] [CrossRef]
- Kitano, M.; Tsujimaru, K.; Anpo, M. Decomposition of water in the separate evolution of hydrogen and oxygen using visible light-responsive TiO2 thin film photocatalysts: Effect of the work function of the substrates on the yield of the reaction. Appl. Catal. A Gen. 2006, 314, 179–183. [Google Scholar] [CrossRef]
- Tode, R.; Ebrahimi, A.; Fukumoto, S.; Iyatani, K.; Takeuchi, M.; Matsuoka, M.; Lee, C.H.; Jiang, C.S.; Anpo, M. Photocatalytic decomposition of water on double-layered visible light-responsive TiO2 thin films prepared by a magnetron sputtering deposition method. Catal. Lett. 2010, 135, 10–15. [Google Scholar] [CrossRef]
- Huang, C.W.; Liao, C.H.; Wu, J.C.S.; Liu, Y.C.; Chang, C.L.; Wu, C.H.; Anpo, M.; Matsuoka, M.; Takeuchi, M. Hydrogen generation from photocatalytic water splitting over TiO2 thin film prepared by electron beam-induced deposition. Int. J. Hydrogen Energy 2010, 35, 12005–12010. [Google Scholar] [CrossRef]
- Liao, C.-H.H.; Huang, C.-W.W.; Wu, J.C.S. Novel dual-layer photoelectrode prepared by RF magnetron sputtering for photocatalytic water splitting. Int. J. Hydrogen Energy 2012, 37, 11632–11639. [Google Scholar] [CrossRef]
- Liao, Y.T.; Huang, C.W.; Liao, C.H.; Wu, J.C.S.; Wu, K.C.W. Synthesis of mesoporous titania thin films (MTTFs) with two different structures as photocatalysts for generating hydrogen from water splitting. Appl. Energy 2012, 100, 75–80. [Google Scholar] [CrossRef]
- Selli, E.; Chiarello, G.L.; Quartarone, E.; Mustarelli, P.; Rossetti, I.; Forni, L. A photocatalytic water splitting device for separate hydrogen and oxygen evolution. Chem. Commun. 2007, 5022–5024. [Google Scholar] [CrossRef]
- Hattori, M.; Noda, K.; Matsushige, K. High-purity hydrogen generation by ultraviolet illumination with the membrane composed of titanium dioxide nanotube array and Pd layer. Appl. Phys. Lett. 2011, 99, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Cha, G.; Altomare, M.; Truong, N.N.; Taccardi, N.; Lee, K.; Schmuki, P. Double-Side Co-Catalytic Activation of Anodic TiO2 Nanotube Membranes with Sputter-Coated Pt for Photocatalytic H2 Generation from Water/Methanol Mixtures. Chem.—Asian J. 2017, 12, 314–323. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Zhang, H.; Chen, Y.; Lu, C.; Wang, J. Photocatalytic hydrogen evolution with simultaneous photocatalytic reforming of biomass by Er3+: YAlO3/Pt-TiO2 membranes under visible light driving. Chem. Eng. J. 2015, 273, 277–285. [Google Scholar] [CrossRef]
- Della Foglia, F.; Chiarello, G.L.; Dozzi, M.V.; Piseri, P.; Bettini, L.G.; Vinati, S.; Ducati, C.; Milani, P.; Selli, E. Hydrogen production by photocatalytic membranes fabricated by supersonic cluster beam deposition on glass fiber filters. Int. J. Hydrogen Energy 2014, 39, 13098–13104. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Bae, E.; Choi, W. Photoactive component-loaded Nafion film as a platform of hydrogen generation: Alternative utilization of a classical sensitizing system. J. Photochem. Photobiol. Chem. 2009, 203, 112–118. [Google Scholar] [CrossRef]
- Wu, M.C.; Sápi, A.; Avila, A.; Szabó, M.; Hiltunen, J.; Huuhtanen, M.; Tóth, G.; Kukovecz, Á.; Kónya, Z.; Keiski, R.; et al. Enhanced Photocatalytic Activity of TiO2 Nanofibers and Their Flexible Composite Films: Decomposition of Organic Dyes and Efficient H2 Generation from Ethanol-Water Mixtures. Nano Res. 2011, 4, 360–369. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Z.; Sun, D.D. Hierarchically multifunctional TiO2 nano-thorn membrane for water purification. Chem. Commun. 2010, 46, 6542–6544. [Google Scholar] [CrossRef]
- Nair, A.K.; Jagadeesh, J.B. TiO2 nanosheet-graphene oxide based photocatalytic hierarchical membrane for water purification. Surf. Coatings Technol. 2017, 320, 259–262. [Google Scholar] [CrossRef]
- Tsydenov, D.E.; Parmon, V.N.; Vorontsov, A.V. Toward the design of asymmetric photocatalytic membranes for hydrogen production: Preparation of TiO2-based membranes and their properties. Int. J. Hydrogen Energy 2012, 37, 11046–11060. [Google Scholar] [CrossRef]
- Tsydenov, D.E.; Vorontsov, A.V. Influence of Nafion loading on hydrogen production in a membrane photocatalytic system. J. Photochem. Photobiol. Chem. 2015, 297, 8–13. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Ku, Y. Effect of solution pH on the adsorption and photocatalytic reaction behaviors of dyes using TiO2 and Nafion-coated TiO2. Aspects 2007, 302, 261–268. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Teixeira, F.C.; de Sá, A.I.; Teixeira, A.P.S.; Ortiz-Martínez, V.M.; Ortiz, A.; Ortiz, I.; Rangel, C.M. New modified Nafion-bisphosphonic acid composite membranes for enhanced proton conductivity and PEMFC performance. Int. J. Hydrogen Energy 2020, 1–10. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Libertino, S.; Nicotera, I.; Kosma, V.; Privitera, V.; Scalese, S. Graphene oxide and titania hybrid Nafion membranes for efficient removal of methyl orange dye from water. Carbon 2015, 82, 489–499. [Google Scholar] [CrossRef]
- D’Angelo, D.; Filice, S.; Libertino, S.; Kosma, V.; Nicotera, I.; Privitera, V.; Scalese, S. Photocatalytic properties of Nafion membranes containing graphene oxide/titania nanocomposites. In Proceedings of the 2014 IEEE 9th Nanotechnology Materials and Devices Conference, NMDC 2014, Aci Castello, Italy, 12–15 October 2014. [Google Scholar]
- Ding, X.; Zhou, S.; Jiang, L.; Yang, H. Preparation, photocatalytic activity and mechanism of nano-Titania/Nafion hybrid membrane. J. Sol-Gel Sci. Technol. 2011, 58, 345–354. [Google Scholar] [CrossRef]
- Vohra, M.S.; Tanaka, K. Enhanced photocatalytic activity of nafion-coated TiO2. Environ. Sci. Technol. 2001, 35, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, W. Photocatalytic reactivities of nafion-coated TiO2 for the degradation of charged organic compounds under UV or visible light. J. Phys. Chem. B 2005, 109, 11667–11674. [Google Scholar] [CrossRef]
- Choi, W. Photocatalytic hydrogen production using surface-modified titania nanoparticles. In Proceedings of the Solar Hydrogen and Nanotechnology II, SPIE, San Diego, CA, USA, 27–30 August 2007. [Google Scholar]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Van Heyst, B. An overview of photocatalyst immobilization methods for air pollution remediation. Chem. Eng. J. 2020, 391, 123490. [Google Scholar] [CrossRef]
- Lugo-Vega, C.S.; Serrano-Rosales, B.; de Lasa, H. Immobilized particle coating for optimum photon and TiO2 utilization in scaled air treatment photo reactors. Appl. Catal. B Environ. 2016, 198, 211–223. [Google Scholar] [CrossRef]
- Huang, N.M.; Chang, B.Y.S.; An’Amt, M.N.; Marlinda, A.R.; Norazriena, Y.; Muhamad, M.R.; Harrison, I.; Lim, H.N.; Chia, C.H. Facile hydrothermal preparation of titanium dioxide decorated reduced graphene oxide nanocomposite. Int. J. Nanomed. 2012, 7, 3379–3387. [Google Scholar] [CrossRef] [Green Version]
- Devrim, Y.; Erkan, S.; Baç, N.; Eroglu, I. Improvement of PEMFC performance with Nafion/inorganic nanocomposite membrane electrode assembly prepared by ultrasonic coating technique. Int. J. Hydrogen Energy 2012, 37, 16748–16758. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Liu, Y.; Lu, H.; Leng, J. The quintuple-shape memory effect in electrospun nanofiber membranes. Smart Mater. Struct. 2013, 22, 085020. [Google Scholar] [CrossRef]
- Kuang, L.; Zhang, W. Enhanced hydrogen production by carbon-doped TiO2 decorated with reduced graphene oxide (rGO) under visible light irradiation. RSC Adv. 2016, 6, 2479–2488. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Ortiz, I. New insights in the performance and reuse of rGO/TiO2 composites for the photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Sher Shah, M.S.A.; Park, A.R.; Zhang, K.; Park, J.H.; Yoo, P.J. Green synthesis of biphasic TiO2-reduced graphene oxide nanocomposites with highly enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2012, 4, 3893–3901. [Google Scholar] [CrossRef] [PubMed]








| Thickness | Solvent-Casting | Spraying | Dip-Coating |
|---|---|---|---|
| Photocatalyst Layer (µm) | n.a. | 12.6 ± 2.6 | 9.8 ± 2.1 |
| Membrane Thickness (µm) | 72.0 ± 0.7 | 165.6 ± 1.6 | 162.5 ± 1.2 |
| Parameter | SC | SP | DP |
|---|---|---|---|
| Turbidity (NTU) | 2.6 ± 0.9 | 7.5 ± 2.5 | 64.3 ± 12.9 |
| Photocatalyst Leaching (%) | 0.2 ± 0.1 | 0.4 ± 0.2 | 4.8 ± 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corredor, J.; Perez-Peña, E.; Rivero, M.J.; Ortiz, I. Performance of rGO/TiO2 Photocatalytic Membranes for Hydrogen Production. Membranes 2020, 10, 218. https://doi.org/10.3390/membranes10090218
Corredor J, Perez-Peña E, Rivero MJ, Ortiz I. Performance of rGO/TiO2 Photocatalytic Membranes for Hydrogen Production. Membranes. 2020; 10(9):218. https://doi.org/10.3390/membranes10090218
Chicago/Turabian StyleCorredor, Juan, Eduardo Perez-Peña, Maria J. Rivero, and Inmaculada Ortiz. 2020. "Performance of rGO/TiO2 Photocatalytic Membranes for Hydrogen Production" Membranes 10, no. 9: 218. https://doi.org/10.3390/membranes10090218