Nanofiltration Membrane Characterization and Application: Extracting Lithium in Lepidolite Leaching Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Separation Equipment
2.2. Membrane Materials
2.3. Filtration of Salt Solutions
2.4. Characterization Methods of Membranes
2.5. Analytical Methods
2.6. Calculation
3. Result and Discussions
3.1. Membrane Characterization
3.1.1. FT-IR
3.1.2. Contact Angle
3.1.3. Zeta Potential
3.1.4. Scanning Electronic Microscope
3.1.5. Atomic Force Microscope
3.1.6. Pore size and Effective Thickness
3.1.7. The Pure Water Permeability
3.2. Retention Experiments
3.2.1. Separation of Li+ and SO42−
3.2.2. Separation of Li+ and Al3+
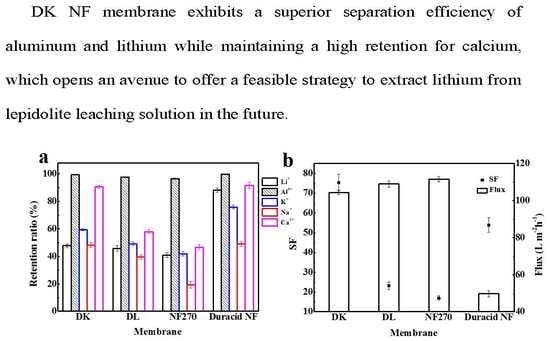
3.2.3. Separation of Multi-Ion System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar]
- Xu, W.; Birbilis, N.; Sha, G.; Wang, Y.; Daniels, J.E.; Xiao, Y.; Ferry, M. A high-specific-strength and corrosion-resistant magnesium alloy. Nat. Mater. 2015, 14, 1229–1235. [Google Scholar]
- Rioja, R.J.; Liu, J. The Evolution of Al-Li Base Products for Aerospace and Space Applications. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 3325–3337. [Google Scholar]
- De Laurentis, N.; Cann, P.M.; Lugt, P.M.; Kadiric, A. The Influence of Base Oil Properties on the Friction Behaviour of Lithium Greases in Rolling/Sliding Concentrated Contacts. Tribol. Lett. 2017, 65, 128. [Google Scholar]
- Choubey, P.K.; Kim, M.; Srivastava, R.R.; Lee, J.; Lee, J. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar]
- Swanson, T.H.; Bailey, S.W. Redetermination of the lepidolite-2M 1 structure. Clays Clay Miner. 1981, 29, 81–90. [Google Scholar]
- Barbosa, L.I.; Gonzalez, J.A.; Ruiz, M.D. Extraction of lithium from beta-spodumene using chlorination roasting with calcium chloride. Thermochim. Acta 2015, 605, 63–67. [Google Scholar]
- Guo, H.; Kuang, G.; Wang, H.; Yu, H.; Zhao, X. Investigation of Enhanced Leaching of Lithium from α-Spodumene Using Hydrofluoric and Sulfuric Acid. Minerals 2017, 7, 205. [Google Scholar]
- Luong, V.T.; Kang, D.J.; An, J.W.; Dao, D.A.; Kim, M.J.; Tran, T. Iron sulphate roasting for extraction of lithium from lepidolite. Hydrometallurgy 2014, 141, 8–16. [Google Scholar]
- Yan, Q.X.; Li, X.H.; Wang, Z.X.; Wu, X.F.; Guo, H.J.; Hu, Q.Y.; Peng, W.J.; Wang, J.X. Extraction of valuable metals from lepidolite. Hydrometallurgy 2012, 117, 116–118. [Google Scholar]
- Mast, E. Lithium Production from Spodumene. Master’s Thesis, McGill University, Montreal, QC, Canada, 1989. [Google Scholar]
- Luong, V.T.; Kang, D.J.; An, J.W.; Kim, M.J.; Tran, T. Factors affecting the extraction of lithium from lepidolite. Hydrometallurgy 2013, 134, 54–61. [Google Scholar]
- Yan, Q.X.; Li, X.H.; Wang, Z.X.; Wang, J.X.; Guo, H.J.; Hu, Q.Y.; Peng, W.J.; Wu, X.F. Extraction of lithium from lepidolite using chlorination roasting–water leaching process. Trans. Nonferrous Met. Soc. China 2012, 22, 1753–1759. [Google Scholar]
- Jia, J.T.; Zhang, Y.W.; Wu, S.; Liao, C.S.; Yan, C.H.; Liu, J.Y.; Deng, G.M.; Rui, X.B. Distribution and Separation of Aluminum in the Rare Earth Solvent Extraction Separation Processes(I). Chin. Rare Earths 2001, 22, 10–13. [Google Scholar]
- Kuang, G.; Guo, H.; Liu, S.; Luo, W.; Shang, Y. Aluminum recovery in leaching solution of lepidolite after lithium extraction. Chin. J. Rare Earths 2014, 38, 102–107. [Google Scholar]
- Wang, L.S.; Wang, F.B.; Wen, X.Q. Study on Impurity Removal Efficiency of the Extraction Process with P204 from Lepidolite Leaching Solution. Rare Met. Cem. Carbides 2013, 41, 24–27. [Google Scholar]
- Xu, M.Y.; Ma, Y.; Sun, X.B.; Zhao, Q.Y. Alum recovery from waterworks sludge by solvent extraction using bi-(2-ethylhexyl) phosphate. J. East China Univ. Sci. Technol. 2007, 33, 369–374. [Google Scholar]
- Bracken, L.; Bruggen BV, D.; Vandecasteele, C. Regeneration of brewery waste water using nanofiltration. Water Res. 2004, 38, 3075–3082. [Google Scholar]
- Zhi, W.; Liu, G.C.; Fan, Z.F.; Yang, X.T.; Wang, J.X.; Wang, S.C. Experimental study on treatment of electroplating wastewater by nanofiltration. J. Membr. Sci. 2007, 305, 185–195. [Google Scholar]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar]
- Somrani, A.; Hamzaoui, A.H.; Pontie, M. Study on lithium separation from salt lake brines by nanofiltration (NF) and low pressure reverse osmosis (LPRO). Desalination 2013, 317, 184–192. [Google Scholar]
- Wen, X.M.; Ma, P.H.; Zhu, C.L.; He, Q.; Deng, X.C. Preliminary study on recovering lithium chloride from lithium-containing waters by nanofiltration. Sep. Purif. Technol. 2006, 49, 230–236. [Google Scholar]
- Reig, M.; Casas, S.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of nanofiltration and bipolar electrodialysis for valorization of seawater desalination brines: Production of drinking and waste water treatment chemicals. Desalination 2016, 382, 13–20. [Google Scholar]
- Bi, Q.; Zhang, Z.; Zhao, C.; Tao, Z. Study on the recovery of lithium from high Mg(2+)/Li(+) ratio brine by nanofiltration. Water Sci. Technol. 2014, 70, 1690. [Google Scholar]
- Badertscher, M.; Bülhmann, P.; Pretsch, E. Structure Determination of Organic Compounds: Tables of Spectral Data; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- López, J.; Reig, M.; Gibert, O.; Torres, E.; Cortina, J.L. Application of nanofiltration for acidic waters containing rare earth elements: Influence of transition elements, acidity and membrane stability. Desalination 2018, 430, 33–44. [Google Scholar]
- Department of Analytical Chemistry, Department of Chemistry, Hangzhou University. Analytical Chemistry Handbook, 2nd ed.; Chemical Industry Press: Beijing, China, 1997. [Google Scholar]
- Fang, J.; Deng, B. Rejection and modeling of arsenate by nanofiltration: Contributions of convection, diffusion and electromigration to arsenic transport. J. Membr. Sci. 2014, 453, 42–51. [Google Scholar]
- Manttari, M.; Pihlajamaki, A.; Nystrom, M. Effect of pH on hydrophilicity and charge and their effect on the filtration efficiency of NF membranes at different pH. J. Membr. Sci. 2006, 280, 311–320. [Google Scholar]
- Shim, Y.; Lee, H.J.; Lee, S.; Moon, S.H.; Cho, J. Effects of Natural Organic Matter and Ionic Species on Membrane Surface Charge. Environ. Sci. Technol. 2002, 36, 3864–3871. [Google Scholar]
- Vrijenhoek, E.M.; Hong, S.; Elimelech, M. Influence of Membrane Surface Properties on Initial Rate of Colloidal Fouling of Reverse Osmosis and Nanofiltration Membranes. J. Membr. Sci. 2001, 188, 115–128. [Google Scholar]
- Van der Bruggen, B.; Schaep, J.; Wilms, D.; Vandecasteele, C. Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J. Membr. Sci. 1999, 156, 29–41. [Google Scholar]
- Bowen, W.R.; Mohammad, A.W. Diafiltration by nanofiltration: Prediction and optimization. AICHE J. 1998, 44, 1799–1812. [Google Scholar]
- Straatsma, J.; Bargeman, G.; Van Der Horst, H.; Wesselingh, J. Can nanofiltration be fully predicted by a model? J. Membr. Sci. 2002, 198, 273–284. [Google Scholar]
- Bargeman, G.; Vollenbroek, J.; Straatsma, J.; Schroën, K.; Boom, R. Nanofiltration of multi-component feeds. Interactions between neutral and charged components and their effect on retention. J. Membr. Sci. 2005, 247, 11–20. [Google Scholar]
- Yao, H.J. Desalination and Concentration of Aqueous Dye Solutions by Nanofiltration Membranes and the Process Similation. Master’s Thesis, Tsinghua University, Beijing, China, 2003. [Google Scholar]
- Gao, L.; Wang, H.Y.; Zhao, Y.J.; Wang, M. The Application of Nanofiltration for Separating Aluminium and Lithium from Lepidolite Leaching Solution. ChemistrySelect 2020, 5, 4979–4987. [Google Scholar]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation—Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar]
- Yaroshchuk, A.E. Non-steric mechanisms of nanofiltration: Superposition of Donnan and dielectric exclusion. Sep. Purif. Technol. 2001, 22, 143–158. [Google Scholar]











| Active Layer | Support Layer | Pressure (MPa) | Membrane Area (m2) | Temperature (K) | pH | |
|---|---|---|---|---|---|---|
| DK | PA | PS | ≤ 4 | 0.38 | ≤ 323 | 2–11 |
| DL | PA | PS | ≤ 4 | 0.38 | ≤ 323 | 2–11 |
| NF270 | PA | PS | ≤ 4 | 0.40 | ≤ 318 | 2–11 |
| Duracid NF | - | - | ≤ 8 | 0.38 | ≤ 343 | < 10 |
| Assignment | Wavenumber (cm−1) | Vibration |
|---|---|---|
| PA (polyamide) | 2934 | CH2 asymmetric stretching |
| 2864 | CH2 attached to O or N stretching/bending | |
| 1650 | C=O stretching (Amide I band) | |
| 1503 | N–H bending | |
| 1485 | CH2 bending | |
| 1410 | C–N stretching coupling with NH2 bending(Amide III band) | |
| 1292 | CONH bending | |
| 690; 714 | N–H out-of-plane bending (Amide IV band) | |
| PS (polysulphone) | 1585 | C=C Phenyl group |
| 1485 | C=C Phenyl group | |
| 1152 | O=S=O symmetric stretching | |
| 1105 | C=C Phenyl group |
| Lp (m·s−1·Pa−1) | Reference | ||
|---|---|---|---|
| This Study | Literatures | ||
| DK | 1.192 × 10−11 | 1.3 × 10−11 | Straatsma [34] |
| DL | 1.815 × 10−11 | 2.1 × 10−11 | Bargeman [35] |
| NF270 | 2.630 × 10−11 | 4.0 × 10−11 | Yao [36] |
| Duracid NF | 5.012 × 10−12 | - | - |
| DK | DL | NF270 | Duracid NF | |
|---|---|---|---|---|
| Contact angle (°) | 36.4 | 34.5 | 25.4 | 35.9 |
| Isoelectric point | 3.49 | 3.69 | 3.33 | - |
| Thickness (μm) | 53.5 | 52.4 | 51.1 | 103.4 |
| Diameter of nodules (nm) | 41.0 | 119.0 | 33.1 | 151.0 |
| Ra (nm) | 4.05 | 12.4 | 4.39 | 7.77 |
| MWCO (Da) | 292.0 | 331.3 | 380.6 | 146.3 |
| rp (nm) | 0.445 | 0.468 | 0.495 | 0.338 |
| Lp (m·s−1·Pa−1) | 1.192 × 10−11 | 1.815 × 10−11 | 2.630 × 10−11 | 5.012 × 10−12 |
| Ions | Ds (10−9 m2·s−1) | rs (nm) | rH (nm) |
|---|---|---|---|
| Li+ | 1.030 | 0.238 | 0.382 |
| Al3+ | - | 0.439 | 0.475 |
| Cl− | 2.032 | 1.21 | 0.332 |
| Na+ | 1.333 | 0.183 | 0.358 |
| K+ | 1.957 | 0.124 | 0.331 |
| Ca2+ | 0.718 | 0.307 | 0.412 |
| SO42− | 1.065 | 0.229 | 0.379 |
| Component | Concentration (mol/L) | Species Name | Concentration (mol/L) | % of Total Concentration |
|---|---|---|---|---|
| Lithium | 0.0471 | Li+ | 0.045544 | 96.698 |
| LiCl (aq) | 0.000314 | 0.667 | ||
| LiSO4− | 0.001241 | 2.635 | ||
| Chlorine | 0.0157 | Cl− | 0.015408 | 97.998 |
| LiCl (aq) | 0.000314 | 2.002 | ||
| Sulfur | 0.0157 | SO42− | 0.014481 | 92.095 |
| LiSO4− | 0.001241 | 7.904 |
| Retention Ratio (%) | Flux (L m−2 h−1) | pH of Permeate | ||
|---|---|---|---|---|
| Li+ | SO42− | |||
| DK | 73.6 | 97.9 | 158.5 | 5.378 |
| DL | 72.9 | 97.4 | 167.8 | 5.436 |
| NF270 | 66.8 | 96.0 | 206.4 | 5.231 |
| Duracid NF | 91.6 | 98.7 | 74.94 | 5.325 |
| Component | Concentration (mol/L) | Species Name | Concentration (mol/L) | % of Total Concentration |
|---|---|---|---|---|
| Lithium | 0.0471 | Li+ | 0.044613 | 94.58 |
| LiCl (aq) | 0.002557 | 5.42 | ||
| Aluminum | 0.0399 | Al3+ | 0.038964 | 97.716 |
| AlOH2+ | 0.000219 | 0.548 | ||
| Al3(OH)45+ | 1.9061 × 10–5 | 0.143 | ||
| Al2(OH)24+ | 0.000113 | 0.565 | ||
| AlCl2+ | 0.000408 | 1.024 | ||
| Al(OH)2+ | 0.00000114 | - | ||
| Al(OH)3 (aq) | 8.1981 × 10–10 | - | ||
| Al(OH)4− | 1.5238 × 10–12 | - | ||
| Chlorine | 0.0157 | Cl− | 0.163830 | 98.222 |
| LiCl (aq) | 0.002557 | 1.533 | ||
| AlCl2+ | 0.000408 | 0.245 |
| Retention Ratio (%) | SF | Flux (L m−2 h−1) | pH of Permeate | ||
|---|---|---|---|---|---|
| Li+ | Al3+ | ||||
| DK | 45.0 | 99.9 | 471. 3 | 103.8 | 3.077 |
| DL | 44.8 | 99.6 | 135.0 | 110.2 | 3.192 |
| NF270 | 39.8 | 97.8 | 27.8 | 115.1 | 2.728 |
| Duracid NF | 90.1 | 99.9 | 218.6 | 54.4 | 3.015 |
| Component | Concentration (mol/L) | Species Name | Concentration (mol/L) | % of Total Concentration |
|---|---|---|---|---|
| Lithium | 0.0471 | Li+ | 0.04462 | 94.596 |
| LiCl (aq) | 0.0025488 | 5.404 | ||
| Aluminum | 0.0399 | Al3+ | 0.038966 | 97.721 |
| AlOH2+ | 0.000218 | 0.548 | ||
| Al3(OH)45+ | 0.000019091 | 0.144 | ||
| Al2(OH)24+ | 0.00011262 | 0.565 | ||
| AlCl2+ | 0.00040686 | 1.020 | ||
| Al(OH)2+ | 1.1377 × 10–6 | - | ||
| Al(OH)3 (aq) | 8.1765 × 10–10 | - | ||
| Al(OH)4− | 1.5225 × 10–12 | - | ||
| Potassium | 0.00338 | K+ | 0.0032418 | 96.026 |
| KCl (aq) | 0.00013415 | 3.974 | ||
| Sodium | 0.00270 | Na+ | 0.0025896 | 96.026 |
| NaCl (aq) | 0.00010716 | 3.974 | ||
| Calcium | 0.000349 | Ca2+ | 0.00031204 | 89.332 |
| CaCl+ | 0.000037263 | 10.668 | ||
| CaOH+ | 6.4718 × 10–14 | - | ||
| Chlorine | 0.0157 | Cl− | 0.16356 | 98.061 |
| NaCl (aq) | 0.00010716 | 0.064 | ||
| AlCl2+ | 0.00040686 | 0.244 | ||
| LiCl (aq) | 0.0025488 | 1.528 | ||
| CaCl+ | 0.000037263 | 0.022 | ||
| KCl (aq) | 0.00013415 | 0.08 |
| Retention Ratio (%) | SF | Flux (L m−2 h−1) | pH of Permeate | |||||
|---|---|---|---|---|---|---|---|---|
| Li+ | Al3+ | K+ | Na+ | Ca2+ | ||||
| DK | 47.7 | 99.3 | 59.4 | 48.3 | 90.6 | 75.4 | 104.5 | 3.054 |
| DL | 45.7 | 97.7 | 49.2 | 39.8 | 58.0 | 23.3 | 109.1 | 3.176 |
| NF270 | 40.9 | 96.5 | 41.9 | 19.4 | 46.8 | 16.9 | 111.6 | 2.720 |
| Duracid NF | 88.2 | 99.8 | 75.8 | 49.0 | 91.7 | 53.9 | 49.9 | 3.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Wang, H.; Zhang, Y.; Wang, M. Nanofiltration Membrane Characterization and Application: Extracting Lithium in Lepidolite Leaching Solution. Membranes 2020, 10, 178. https://doi.org/10.3390/membranes10080178
Gao L, Wang H, Zhang Y, Wang M. Nanofiltration Membrane Characterization and Application: Extracting Lithium in Lepidolite Leaching Solution. Membranes. 2020; 10(8):178. https://doi.org/10.3390/membranes10080178
Chicago/Turabian StyleGao, Lin, Huaiyou Wang, Yue Zhang, and Min Wang. 2020. "Nanofiltration Membrane Characterization and Application: Extracting Lithium in Lepidolite Leaching Solution" Membranes 10, no. 8: 178. https://doi.org/10.3390/membranes10080178