Macropinocytosis in Different Cell Types: Similarities and Differences
Abstract
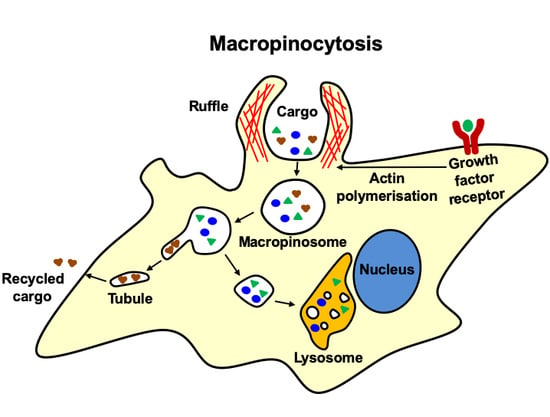
:1. Introduction
Analysing Macropinocytosis
2. Non-Mammalian Systems
3. Mammalian Cells
3.1. Immune Cells
3.1.1. Dendritic Cells
Role of Macropinocytosis in Antigen Presentation by DCs
Constitutive Macropinocytosis in DCs and the Effect of Activating Stimuli
Molecular Machinery Involved in Macropinocytosis by DCs
3.1.2. Macrophages
Constitutive and Activated Macropinocytosis in Macrophages
Membrane Ruffling, Macropinocytosis and Signalling in Macrophages
Effect of Macrophage Functional State on Macropinocytosis
Macropinosome Maturation in Macrophages
Functions of Macropinocytosis in Macrophages
3.1.3. B Cells and T Cells
3.2. Endothelial and Epithelial Cells
3.2.1. Endothelial Cells
3.2.2. Epithelial Cells
3.3. Fibroblasts
3.4. Cells of the Nervous System
3.4.1. Neurons
3.4.2. Microglia
3.5. Cancer Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.A.; King, J.S. The breadth of macropinocytosis research. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2019, 374, 20180146. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.A. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, M.C.; Teasdale, R.D. Defining macropinocytosis. Traffic 2009, 10, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009, 11, 510–520. [Google Scholar] [CrossRef]
- Lewis, W.H. Pinocytosis by Malignant Cells. Am. J. Cancer 1937, 29, 666–679. [Google Scholar]
- Swanson, J.A.; Watts, C. Macropinocytosis. Trends Cell Biol. 1995, 5, 424–428. [Google Scholar] [CrossRef]
- Lim, J.P.; Gosavi, P.; Mintern, J.D.; Ross, E.M.; Gleeson, P.A. Sorting nexin 5 selectively regulates dorsal-ruffle-mediated macropinocytosis in primary macrophages. J. Cell Sci. 2015, 128, 4407–4419. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.T.; Teasdale, R.D.; Liebl, D. Macropinosome quantitation assay. MethodsX 2014, 1, 36–41. [Google Scholar] [CrossRef]
- West, M.A.; Bretscher, M.S.; Watts, C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 1989, 109, 2731–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallusto, F.; Cella, M.; Danieli, C.; Lanzavecchia, A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: Downregulation by cytokines and bacterial products. J. Exp. Med. 1995, 182, 389–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, M.A.; Prescott, A.R.; Eskelinen, E.L.; Ridley, A.J.; Watts, C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr. Biol. 2000, 10, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Norbury, C.C.; Chambers, B.J.; Prescott, A.R.; Ljunggren, H.G.; Watts, C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur. J. Immunol. 1997, 27, 280–288. [Google Scholar] [CrossRef]
- Norbury, C.C.; Hewlett, L.J.; Prescott, A.R.; Shastri, N.; Watts, C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity 1995, 3, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B.; et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013, 497, 633–637. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.P.; Teasdale, R.D.; Gleeson, P.A. SNX5 is essential for efficient macropinocytosis and antigen processing in primary macrophages. Biol. Open 2012, 1, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Racoosin, E.L.; Swanson, J.A. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J. Cell Biol. 1993, 121, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Araki, N. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 1996, 135, 1249–1260. [Google Scholar] [CrossRef] [Green Version]
- Wennstrom, S.; Hawkins, P.; Cooke, F.; Hara, K.; Yonezawa, K.; Kasuga, M.; Jackson, T.; Claesson-Welsh, L.; Stephens, L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr. Biol. 1994, 4, 385–393. [Google Scholar] [CrossRef]
- Toh, W.H.; Louber, J.; Mahmoud, I.S.; Chia, J.; Bass, G.T.; Dower, S.K.; Verhagen, A.M.; Gleeson, P.A. FcRn mediates fast recycling of endocytosed albumin and IgG from early macropinosomes in primary macrophages. J. Cell Sci. 2019, 133, jcs235416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norbury, C.C. Drinking a lot is good for dendritic cells. Immunology 2006, 117, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Roche, P.A. Macropinocytosis in phagocytes: Regulation of MHC class-II-restricted antigen presentation in dendritic cells. Front. Physiol. 2015, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Commisso, C.; Flinn, R.J.; Bar-Sagi, D. Determining the macropinocytic index of cells through a quantitative image-based assay. Nat. Protoc. 2014, 9, 182–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platt, C.D.; Ma, J.K.; Chalouni, C.; Ebersold, M.; Bou-Reslan, H.; Carano, R.A.; Mellman, I.; Delamarre, L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc. Natl. Acad. Sci. USA 2010, 107, 4287–4292. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wan, T.; Wan, M.; Liu, B.; Cheng, R.; Zhang, R. The effect of the size of fluorescent dextran on its endocytic pathway. Cell Biol. Int. 2015, 39, 531–539. [Google Scholar] [CrossRef]
- Ivanov, A.I. Pharmacological inhibition of endocytic pathways: Is it specific enough to be useful? In Exocytosis and Endocytosis; Springer: Berlin/Heidelberg, Germany, 2008; pp. 15–33. [Google Scholar]
- West, M.A.; Wallin, R.P.; Matthews, S.P.; Svensson, H.G.; Zaru, R.; Ljunggren, H.-G.; Prescott, A.R.; Watts, C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science 2004, 305, 1153–1157. [Google Scholar] [CrossRef]
- Peterson, J.R.; Mitchison, T.J. Small molecules, big impact: A history of chemical inhibitors and the cytoskeleton. Chem. Biol. 2002, 9, 1275–1285. [Google Scholar] [CrossRef] [Green Version]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef] [Green Version]
- Canton, J. Macropinocytosis: New Insights Into Its Underappreciated Role in Innate Immune Cell Surveillance. Front. Immunol. 2018, 9, 2286. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.D.; Kay, R.R. The physiological regulation of macropinocytosis during Dictyostelium growth and development. J. Cell. Sci. 2018, 131, jcs213736. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.D.; Paschke, P.I.; Kay, R.R. Function of small GTPases in Dictyostelium macropinocytosis. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2019, 374, 20180150. [Google Scholar] [CrossRef] [Green Version]
- Veltman, D.M.; Williams, T.D.; Bloomfield, G.; Chen, B.C.; Betzig, E.; Insall, R.H.; Kay, R.R. A plasma membrane template for macropinocytic cups. Elife 2016, 5, e20085. [Google Scholar] [CrossRef]
- Fares, H.; Greenwald, I. Genetic analysis of endocytosis in Caenorhabditis elegans: Coelomocyte uptake defective mutants. Genetics 2001, 159, 133–145. [Google Scholar]
- Maekawa, M.; Terasaka, S.; Mochizuki, Y.; Kawai, K.; Ikeda, Y.; Araki, N.; Skolnik, E.Y.; Taguchi, T.; Arai, H. Sequential breakdown of 3-phosphorylated phosphoinositides is essential for the completion of macropinocytosis. Proc. Natl. Acad. Sci. USA 2014, 111, E978–E987. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Cheng, D.; Chu, J.; Zhang, T.; Dong, Z.; Lou, H.; Zhu, L.; Liu, Y. A Novel Method to Image Macropinocytosis in Vivo. Front. Neurosci. 2018, 12, 324. [Google Scholar] [CrossRef] [Green Version]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Sathe, P.; Shortman, K. The steady-state development of splenic dendritic cells. Mucosal Immunol. 2008, 1, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef]
- Sallusto, F. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Von Delwig, A.; Hilkens, C.M.; Altmann, D.M.; Holmdahl, R.; Isaacs, J.D.; Harding, C.V.; Robertson, H.; McKie, N.; Robinson, J.H. Inhibition of macropinocytosis blocks antigen presentation of type II collagen in vitro and in vivo in HLA-DR1 transgenic mice. Arthritis Res. Ther. 2006, 8, R93. [Google Scholar] [CrossRef] [Green Version]
- Diken, M.; Kreiter, S.; Selmi, A.; Britten, C.M.; Huber, C.; Tureci, O.; Sahin, U. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 2011, 18, 702–708. [Google Scholar] [CrossRef] [Green Version]
- Moreau, H.D.; Blanch-Mercader, C.; Attia, R.; Maurin, M.; Alraies, Z.; Sanseau, D.; Malbec, O.; Delgado, M.G.; Bousso, P.; Joanny, J.F.; et al. Macropinocytosis Overcomes Directional Bias in Dendritic Cells Due to Hydraulic Resistance and Facilitates Space Exploration. Dev. Cell 2019, 49, 171–188.e5. [Google Scholar] [CrossRef]
- Canton, J.; Schlam, D.; Breuer, C.; Gutschow, M.; Glogauer, M.; Grinstein, S. Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of NOD2 ligands in macrophages. Nat. Commun. 2016, 7, 11284. [Google Scholar] [CrossRef] [Green Version]
- Bohdanowicz, M.; Schlam, D.; Hermansson, M.; Rizzuti, D.; Fairn, G.D.; Ueyama, T.; Somerharju, P.; Du, G.; Grinstein, S. Phosphatidic acid is required for the constitutive ruffling and macropinocytosis of phagocytes. Mol. Biol. Cell 2013, 24, 1700–1712. [Google Scholar] [CrossRef]
- Garrett, W.S.; Chen, L.M.; Kroschewski, R.; Ebersold, M.; Turley, S.; Trombetta, S.; Galan, J.E.; Mellman, I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 2000, 102, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Redka, D.S.; Gütschow, M.; Grinstein, S.; Canton, J. Differential ability of proinflammatory and anti-inflammatory macrophages to perform macropinocytosis. Mol. Biol. Cell 2018, 29, 53–65. [Google Scholar] [CrossRef]
- Egami, Y.; Taguchi, T.; Maekawa, M.; Arai, H.; Araki, N. Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation. Front. Physiol. 2014, 5, 374. [Google Scholar] [CrossRef] [Green Version]
- Hackstein, H.; Taner, T.; Logar, A.J.; Thomson, A.W. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood 2002, 100, 1084–1087. [Google Scholar] [CrossRef] [Green Version]
- De Baey, A.; Lanzavecchia, A. The role of aquaporins in dendritic cell macropinocytosis. J. Exp. Med. 2000, 191, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Calmette, J.; Bertrand, M.; Vetillard, M.; Ellouze, M.; Flint, S.; Nicolas, V.; Biola-Vidamment, A.; Pallardy, M.; Morand, E.; Bachelerie, F.; et al. Glucocorticoid-Induced Leucine Zipper Protein Controls Macropinocytosis in Dendritic Cells. J. Immunol. 2016, 197, 4247–4256. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Condon, N.D.; Heddleston, J.M.; Chew, T.L.; Luo, L.; McPherson, P.S.; Ioannou, M.S.; Hodgson, L.; Stow, J.L.; Wall, A.A. Macropinosome formation by tent pole ruffling in macrophages. J. Cell Biol. 2018, 217, 3873–3885. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.P.; Singla, B.; Ghoshal, P.; Faulkner, J.L.; Cherian-Shaw, M.; O’Connor, P.M.; She, J.X.; Belin de Chantemele, E.J.; Csanyi, G. Identification of novel macropinocytosis inhibitors using a rational screen of Food and Drug Administration-approved drugs. Br. J. Pharmacol. 2018, 175, 3640–3655. [Google Scholar] [CrossRef] [Green Version]
- Anzinger, J.J.; Chang, J.; Xu, Q.; Buono, C.; Li, Y.; Leyva, F.J.; Park, B.C.; Greene, L.E.; Kruth, H.S. Native low-density lipoprotein uptake by macrophage colony-stimulating factor-differentiated human macrophages is mediated by macropinocytosis and micropinocytosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2022–2031. [Google Scholar] [CrossRef] [Green Version]
- Corrotte, M.; Chasserot-Golaz, S.; Huang, P.; Du, G.; Ktistakis, N.T.; Frohman, M.A.; Vitale, N.; Bader, M.F.; Grant, N.J. Dynamics and function of phospholipase D and phosphatidic acid during phagocytosis. Traffic 2006, 7, 365–377. [Google Scholar] [CrossRef]
- Bohdanowicz, M.; Grinstein, S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol. Rev. 2013, 93, 69–106. [Google Scholar] [CrossRef] [Green Version]
- Welliver, T.P.; Swanson, J.A. A growth factor signaling cascade confined to circular ruffles in macrophages. Biol. Open 2012, 1, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Welliver, T.P.; Chang, S.L.; Linderman, J.J.; Swanson, J.A. Ruffles limit diffusion in the plasma membrane during macropinosome formation. J. Cell. Sci. 2011, 124, 4106–4114. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Pacitto, R.; Yao, Y.; Inoki, K.; Swanson, J.A. Growth factor signaling to mTORC1 by amino acid-laden macropinosomes. J. Cell Biol. 2015, 211, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Wall, A.A.; Luo, L.; Hung, Y.; Tong, S.J.; Condon, N.D.; Blumenthal, A.; Sweet, M.J.; Stow, J.L. Small GTPase Rab8a-recruited Phosphatidylinositol 3-Kinase gamma Regulates Signaling and Cytokine Outputs from Endosomal Toll-like Receptors. J. Biol. Chem. 2017, 292, 4411–4422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, A.A.; Condon, N.D.; Luo, L.; Stow, J.L. Rab8a localisation and activation by Toll-like receptors on macrophage macropinosomes. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2019, 374, 20180151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, M.C.; Lindsay, M.R.; Luetterforst, R.; Hamilton, N.; Simpson, F.; Parton, R.G.; Gleeson, P.A.; Teasdale, R.D. Visualisation of macropinosome maturation by the recruitment of sorting nexins. J. Cell Sci. 2006, 119, 3967–3980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marechal, V.; Prevost, M.C.; Petit, C.; Perret, E.; Heard, J.M.; Schwartz, O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 2001, 75, 11166–11177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, F.D.; Harwood, N.E. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 2009, 9, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, B.E.; De la Cruz-Lopez, J.J.; Castaneda-Sanchez, J.I.; Munoz-Duarte, A.R.; Hernandez-Perez, A.D.; Villegas-Castrejon, H.; Garcia-Latorre, E.; Caamal-Ley, A.; Luna-Herrera, J. Macropinocytosis is responsible for the uptake of pathogenic and non-pathogenic mycobacteria by B lymphocytes(Raji cells). BMC Microbiol. 2012, 12, 246. [Google Scholar] [CrossRef] [Green Version]
- Charpentier, J.C.; Chen, D.; Lapinski, P.E.; Turner, J.; Grigorova, I.; Swanson, J.A.; King, P.D. Macropinocytosis drives T cell growth by sustaining the activation of mTORC1. Nat. Commun. 2020, 11, 180. [Google Scholar] [CrossRef]
- Fazil, M.H.; Ong, S.T.; Chalasani, M.L.; Low, J.H.; Kizhakeyil, A.; Mamidi, A.; Lim, C.F.; Wright, G.D.; Lakshminarayanan, R.; Kelleher, D.; et al. GapmeR cellular internalization by macropinocytosis induces sequence-specific gene silencing in human primary T-cells. Sci. Rep. 2016, 6, 37721. [Google Scholar] [CrossRef]
- Frecha, C.; Levy, C.; Costa, C.; Negre, D.; Amirache, F.; Buckland, R.; Russell, S.J.; Cosset, F.L.; Verhoeyen, E. Measles virus glycoprotein-pseudotyped lentiviral vector-mediated gene transfer into quiescent lymphocytes requires binding to both SLAM and CD46 entry receptors. J. Virol. 2011, 85, 5975–5985. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.Q.; Lossinsky, A.S.; Popik, W.; Li, X.; Gujuluva, C.; Kriederman, B.; Roberts, J.; Pushkarsky, T.; Bukrinsky, M.; Witte, M.; et al. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J. Virol. 2002, 76, 6689–6700. [Google Scholar] [CrossRef] [Green Version]
- Raghu, H.; Sharma-Walia, N.; Veettil, M.V.; Sadagopan, S.; Chandran, B. Kaposi’s sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. J. Virol. 2009, 83, 4895–4911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, L.N.; McCarthy, E.M.C.; Narang, P.; Khan, N.A.; Ward, T.H. Escherichia coli K1 utilizes host macropinocytic pathways for invasion of brain microvascular endothelial cells. Traffic 2017, 18, 733–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajikumar, A.; Long, M.B.; Heath, P.R.; Wharton, S.B.; Ince, P.G.; Ridger, V.C.; Simpson, J.E. Neutrophil-Derived Microvesicle Induced Dysfunction of Brain Microvascular Endothelial Cells In Vitro. Int. J. Mol. Sci. 2019, 20, 5227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Li, J.; Jang, C.; Arany, Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017, 36, 2321–2333. [Google Scholar] [CrossRef]
- Martin-Ramirez, J.; Hofman, M.; van den Biggelaar, M.; Hebbel, R.P.; Voorberg, J. Establishment of outgrowth endothelial cells from peripheral blood. Nat. Protoc. 2012, 7, 1709–1715. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, K.; Llorente, A.; Rodal, S.K.; Eker, P.; Garred, O.; Stahlhut, M.; van Deurs, B. Apical macropinocytosis in polarized MDCK cells: Regulation by N-ethylmaleimide-sensitive proteins. Eur. J. Cell Biol. 2000, 79, 447–457. [Google Scholar] [CrossRef]
- Mettlen, M.; Platek, A.; Van Der Smissen, P.; Carpentier, S.; Amyere, M.; Lanzetti, L.; de Diesbach, P.; Tyteca, D.; Courtoy, P.J. Src triggers circular ruffling and macropinocytosis at the apical surface of polarized MDCK cells. Traffic 2006, 7, 589–603. [Google Scholar] [CrossRef]
- Hewlett, L.J.; Prescott, A.R.; Watts, C. The coated pit and macropinocytic pathways serve distinct endosome populations. J. Cell Biol. 1994, 124, 689–703. [Google Scholar] [CrossRef] [Green Version]
- Liberali, P.; Kakkonen, E.; Turacchio, G.; Valente, C.; Spaar, A.; Perinetti, G.; Böckmann, R.A.; Corda, D.; Colanzi, A.; Marjomaki, V.; et al. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J. 2008, 27, 970–981. [Google Scholar] [CrossRef] [Green Version]
- Nakase, I.; Tadokoro, A.; Kawabata, N.; Takeuchi, T.; Katoh, H.; Hiramoto, K.; Negishi, M.; Nomizu, M.; Sugiura, Y.; Futaki, S. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry 2007, 46, 492–501. [Google Scholar] [CrossRef]
- Chung, J.J.; Huber, T.B.; Godel, M.; Jarad, G.; Hartleben, B.; Kwoh, C.; Keil, A.; Karpitskiy, A.; Hu, J.; Huh, C.J.; et al. Albumin-associated free fatty acids induce macropinocytosis in podocytes. J. Clin. Investig. 2015, 125, 2307–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Perez, B.E.; Hernandez-Gonzalez, J.C.; Garcia-Nieto, S.; Luna-Herrera, J. Internalization of a non-pathogenic mycobacteria by macropinocytosis in human alveolar epithelial A549 cells. Microb. Pathog. 2008, 45, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.L.; Ryan, T.A.; Jones, B.D.; Smith, S.J.; Falkow, S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 1993, 364, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Zenni, M.K.; Giardina, P.C.; Harvey, H.A.; Shao, J.; Ketterer, M.R.; Lubaroff, D.M.; Williams, R.D.; Apicella, M.A. Macropinocytosis as a mechanism of entry into primary human urethral epithelial cells by Neisseria gonorrhoeae. Infect. Immunity 2000, 68, 1696–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulherkar, N.; Raaben, M.; de la Torre, J.C.; Whelan, S.P.; Chandran, K. The Ebola virus glycoprotein mediates entry via a non-classical dynamin-dependent macropinocytic pathway. Virology 2011, 419, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Krieger, S.E.; Kim, C.; Zhang, L.; Marjomaki, V.; Bergelson, J.M. Echovirus 1 entry into polarized Caco-2 cells depends on dynamin, cholesterol, and cellular factors associated with macropinocytosis. J. Virol. 2013, 87, 8884–8895. [Google Scholar] [CrossRef] [Green Version]
- Mercer, J.; Knébel, S.; Schmidt, F.I.; Crouse, J.; Burkard, C.; Helenius, A. Vaccinia virus strains use distinct forms of macropinocytosis for host-cell entry. Proc. Natl. Acad. Sci. USA 2010, 107, 9346–9351. [Google Scholar] [CrossRef] [Green Version]
- Rossman, J.S.; Leser, G.P.; Lamb, R.A. Filamentous influenza virus enters cells via macropinocytosis. J. Virol. 2012, 86, 10950–10960. [Google Scholar] [CrossRef] [Green Version]
- Dou, D.; Revol, R.; Ostbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef]
- Ridley, A.J.; Paterson, H.F.; Johnston, C.L.; Diekmann, D.; Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992, 70, 401–410. [Google Scholar] [CrossRef]
- Mellström, K.; Heldin, C.H.; Westermark, B. Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp. Cell. Res. 1988, 177, 347–359. [Google Scholar] [CrossRef]
- Dharmawardhane, S.; Schurmann, A.; Sells, M.A.; Chernoff, J.; Schmid, S.L.; Bokoch, G.M. Regulation of macropinocytosis by p21-activated kinase-1. Mol. Biol. Cell 2000, 11, 3341–3352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suetsugu, S.; Yamazaki, D.; Kurisu, S.; Takenawa, T. Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev. Cell 2003, 5, 595–609. [Google Scholar] [CrossRef] [Green Version]
- Zeineddine, R.; Yerbury, J.J. The role of macropinocytosis in the propagation of protein aggregation associated with neurodegenerative diseases. Front. Physiol. 2015, 6, 277. [Google Scholar] [CrossRef] [Green Version]
- Holmes, B.B.; DeVos, S.L.; Kfoury, N.; Li, M.; Jacks, R.; Yanamandra, K.; Ouidja, M.O.; Brodsky, F.M.; Marasa, J.; Bagchi, D.P.; et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA 2013, 110, E3138–E3147. [Google Scholar] [CrossRef] [Green Version]
- Evans, L.D.; Wassmer, T.; Fraser, G.; Smith, J.; Perkinton, M.; Billinton, A.; Livesey, F.J. Extracellular Monomeric and Aggregated Tau Efficiently Enter Human Neurons through Overlapping but Distinct Pathways. Cell Rep. 2018, 22, 3612–3624. [Google Scholar] [CrossRef] [Green Version]
- Zeineddine, R.; Pundavela, J.F.; Corcoran, L.; Stewart, E.M.; Do-Ha, D.; Bax, M.; Guillemin, G.; Vine, K.L.; Hatters, D.M.; Ecroyd, H.; et al. SOD1 protein aggregates stimulate macropinocytosis in neurons to facilitate their propagation. Mol. Neurodegener. 2015, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Tam, J.H.; Seah, C.; Chiu, J.; Tyrer, A.; Cregan, S.P.; Meakin, S.O.; Pasternak, S.H. Arf6 controls beta-amyloid production by regulating macropinocytosis of the Amyloid Precursor Protein to lysosomes. Mol. Brain 2015, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Kabayama, H.; Nakamura, T.; Takeuchi, M.; Iwasaki, H.; Taniguchi, M.; Tokushige, N.; Mikoshiba, K. Ca2+ induces macropinocytosis via F-actin depolymerization during growth cone collapse. Mol. Cell Neurosci. 2009, 40, 27–38. [Google Scholar] [CrossRef]
- Kabayama, H.; Takeuchi, M.; Taniguchi, M.; Tokushige, N.; Kozaki, S.; Mizutani, A.; Nakamura, T.; Mikoshiba, K. Syntaxin 1B suppresses macropinocytosis and semaphorin 3A-induced growth cone collapse. J. Neurosci. 2011, 31, 7357–7364. [Google Scholar] [CrossRef]
- Jin, Z.; Strittmatter, S.M. Rac1 mediates collapsin-1-induced growth cone collapse. J. Neurosci. 1997, 17, 6256–6263. [Google Scholar] [CrossRef] [PubMed]
- Tom, V.J.; Steinmetz, M.P.; Miller, J.H.; Doller, C.M.; Silver, J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 2004, 24, 6531–6539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Ranson, P.A.; Thomas, W.E. Pinocytosis as a select marker of ramified microglia in vivo and in vitro. J. Histochem. Cytochem. 1991, 39, 853–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandrekar, S.; Jiang, Q.; Lee, C.Y.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Landreth, G.E. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J. Neurosci. 2009, 29, 4252–4262. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, C.; Tiberi, A.; Giustizieri, M.; Marrone, M.C.; Gobbo, F.; Carucci, N.M.; Meli, G.; Arisi, I.; D’Onofrio, M.; Marinelli, S.; et al. NGF steers microglia toward a neuroprotective phenotype. Glia 2018, 66, 1395–1416. [Google Scholar] [CrossRef] [Green Version]
- Fitzner, D.; Schnaars, M.; van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.K.; Simons, M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011, 124, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Kamphorst, J.J.; Nofal, M.; Commisso, C.; Hackett, S.R.; Lu, W.; Grabocka, E.; Vander Heiden, M.G.; Miller, G.; Drebin, J.A.; Bar-Sagi, D.; et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015, 75, 544–553. [Google Scholar] [CrossRef] [Green Version]
- Ha, K.D.; Bidlingmaier, S.M.; Liu, B. Macropinocytosis Exploitation by Cancers and Cancer Therapeutics. Front. Physiol. 2016, 7, 381. [Google Scholar] [CrossRef] [Green Version]
- Recouvreux, M.V.; Commisso, C. Macropinocytosis: A Metabolic Adaptation to Nutrient Stress in Cancer. Front. Endocrinol. (Lausanne) 2017, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Commisso, C. Macropinocytosis in Cancer: A Complex Signaling Network. Trends Cancer 2019, 5, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, X.; Liu, Y.; Li, Y.; Colvin, R.A.; Tong, L.; Wu, S.; Chen, X. Extracellular ATP is internalized by macropinocytosis and induces intracellular ATP increase and drug resistance in cancer cells. Cancer Lett. 2014, 351, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Palm, W.; Park, Y.; Wright, K.; Pavlova, N.N.; Tuveson, D.A.; Thompson, C.B. The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell 2015, 162, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Pacitto, R.; Inoki, K.; Swanson, J. Macropinocytosis, mTORC1 and cellular growth control. Cell Mol. Life Sci. 2018, 75, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- Redelman-Sidi, G.; Binyamin, A.; Gaeta, I.; Palm, W.; Thompson, C.B.; Romesser, P.B.; Lowe, S.W.; Bagul, M.; Doench, J.G.; Root, D.E.; et al. The Canonical Wnt Pathway Drives Macropinocytosis in Cancer. Cancer Res. 2018, 78, 4658–4670. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, K.; Nakayama, Y.; Sato, I.; Ikeda, K.; Hoshino, M.; Endo, T.; Yamaguchi, N. Role of Src-family kinases in formation and trafficking of macropinosomes. J. Cell Physiol. 2007, 211, 220–232. [Google Scholar] [CrossRef]
- Veithen, A.; Cupers, P.; Baudhuin, P.; Courtoy, P.J. v-Src induces constitutive macropinocytosis in rat fibroblasts. J. Cell Sci. 1996, 109, 2005–2012. [Google Scholar]
- Overmeyer, J.H.; Kaul, A.; Johnson, E.E.; Maltese, W.A. Active ras triggers death in glioblastoma cells through hyperstimulation of macropinocytosis. Mol. Cancer Res. 2008, 6, 965–977. [Google Scholar] [CrossRef] [Green Version]
- Fennell, M.; Commisso, C.; Ramirez, C.; Garippa, R.; Bar-Sagi, D. High-content, full genome siRNA screen for regulators of oncogenic HRAS-driven macropinocytosis. Assay Drug Dev. Technol. 2015, 13, 347–355. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.P.; Mintern, J.D.; Gleeson, P.A. Macropinocytosis in Different Cell Types: Similarities and Differences. Membranes 2020, 10, 177. https://doi.org/10.3390/membranes10080177
Lin XP, Mintern JD, Gleeson PA. Macropinocytosis in Different Cell Types: Similarities and Differences. Membranes. 2020; 10(8):177. https://doi.org/10.3390/membranes10080177
Chicago/Turabian StyleLin, Xiao Peng, Justine D. Mintern, and Paul A. Gleeson. 2020. "Macropinocytosis in Different Cell Types: Similarities and Differences" Membranes 10, no. 8: 177. https://doi.org/10.3390/membranes10080177