New Perspectives on Fuel Cell Technology: A Brief Review
Abstract
:1. Introduction
2. Materials and Methods
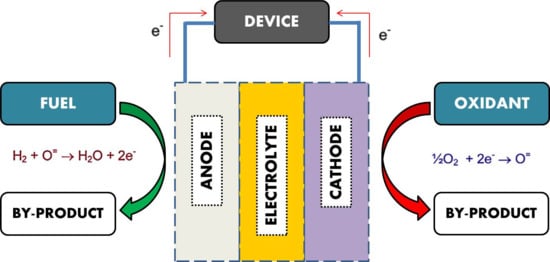
2.1. Fuel Cell Varieties and Development
2.2. Direct Methanol Fuel Cell (DMFCs)
Membrane Electrode Assembly (MEA)
2.3. Polymer Electrolyte Membrane Fuel Cell (PEMFCs)
2.4. Solid Oxide Fuel Cell (SOFCs)
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Senjyu, T.; Howlader, A.M. Operational aspects of distribution systems with massive DER penetrations. Integr. Distrib. Energy Resour. Power Syst. 2016, 51–76. [Google Scholar] [CrossRef]
- Bilgili, M.; Özbek, A.; Sahin, B.; Kahraman, A. An overview of renewable electric power capacity and progress in new technologies in the world. Renew. Sustain. Energy Rev. 2015, 49, 323–334. [Google Scholar] [CrossRef]
- Ajanovic, A.; Haas, R. Economic prospects and policy framework for hydrogen as fuel in the transport sector. Energy Policy 2018, 123, 280–288. [Google Scholar] [CrossRef]
- Hames, Y.; Kaya, K.; Baltacioğlu, E.; Turksoy, A. Analysis of the control strategies for fuel saving in the hydrogen fuel cell vehicles. Int. J. Hydrog. Energy 2018, 43, 10810–10821. [Google Scholar] [CrossRef]
- Salleh, M.T.; Jaafar, J.; Mohamed, M.A.; Norddin, M.; Ismail, A.F.; Othman, M.; Rahman, M.A.; Yusof, N.; Aziz, F.; Salleh, W.N.W. Stability of SPEEK/Cloisite®/TAP nanocomposite membrane under Fenton reagent condition for direct methanol fuel cell application. Polym. Degrad. Stab. 2017, 137, 83–99. [Google Scholar] [CrossRef]
- Kim, D.J.; Jo, M.J.; Nam, S.Y. A review of polymer–nanocomposite electrolyte membranes for fuel cell application. J. Ind. Eng. Chem. 2015, 21, 36–52. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrog. Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Wu, J.; Zhao, C.; Jia, Q.; Lew, C.M.; Zhang, L.; Zhang, Y.; Han, M.; Zhu, J.; et al. A facile approach to prepare self-cross-linkable sulfonated poly(ether ether ketone) membranes for direct methanol fuel cells. J. Power Sources 2010, 195, 8061–8066. [Google Scholar] [CrossRef]
- Kim, K.; Heo, P.; Han, J.; Kim, J.; Lee, J.-C. End-group cross-linked sulfonated poly(arylene ether sulfone) via thiol-ene click reaction for high-performance proton exchange membrane. J. Power Sources 2018, 401, 20–28. [Google Scholar] [CrossRef]
- Pan, L.; Ott, S.; Dionigi, F.; Strasser, P. Current challenges related to the deployment of shape-controlled Pt alloy oxygen reduction reaction nanocatalysts into low Pt-loaded cathode layers of proton exchange membrane fuel cells. Curr. Opin. Electrochem. 2019, 18, 61–71. [Google Scholar] [CrossRef]
- Liu, X.; Reddi, K.; Elgowainy, A.; Lohse-Busch, H.; Wang, M.; Rustagi, N. Comparison of well-to-wheels energy use and emissions of a hydrogen fuel cell electric vehicle relative to a conventional gasoline-powered internal combustion engine vehicle. Int. J. Hydrog. Energy 2020, 45, 972–983. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, X.; Chen, B.; Liu, X. Coordination control strategy for the air management of heavy vehicle fuel cell engine. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Suhaili, S.B.H.; Kamal, I.; Shaikh, S.P.S.; Dawood, M.K.; Azad, A.K. Nanostructured graphene materials utilization in fuel cells and batteries: A review. J. Energy Storage 2020, 29, 101386. [Google Scholar] [CrossRef]
- Haghi, E.; Shamsi, H.; Dimitrov, S.; Fowler, M.; Raahemifar, K. Assessing the potential of fuel cell-powered and battery-powered forklifts for reducing GHG emissions using clean surplus power; a game theory approach. Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Inci, M.; Turksoy, O. Review of fuel cells to grid interface: Configurations, technical challenges and trends. J. Clean. Prod. 2019, 213, 1353–1370. [Google Scholar] [CrossRef]
- Widera, B. Renewable hydrogen implementations for combined energy storage, transportation and stationary applications. Therm. Sci. Eng. Prog. 2020, 16, 100460. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Siwal, S.S.; Thakur, S.; Zhang, Q.; Thakur, V. Electrocatalysts for electrooxidation of direct alcohol fuel cell: Chemistry and applications. Mater. Today Chem. 2019, 14, 100182. [Google Scholar] [CrossRef]
- Mallick, R.; Thombre, S.B.; Shrivastava, N.K. Vapor feed direct methanol fuel cells (DMFCs): A review. Renew. Sustain. Energy Rev. 2016, 56, 51–74. [Google Scholar] [CrossRef]
- Jaafar, J.; Ismail, A.F.; Matsuura, T.; Nagai, K. Performance of SPEEK based polymer–nanoclay inorganic membrane for DMFC. J. Membr. Sci. 2011, 382, 202–211. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Achmad, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrog. Energy 2009, 34, 6902–6916. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Daud, W.R.W.; Ho, S.L.; Hasran, U. Overview on the challenges and developments of micro-direct methanol fuel cells (DMFC). J. Power Sources 2007, 163, 743–754. [Google Scholar] [CrossRef]
- Asensio, F.J.; Martin, J.I.S.; Zamora, I.; Saldaña, G.; Oñederra, O. Analysis of electrochemical and thermal models and modeling techniques for polymer electrolyte membrane fuel cells. Renew. Sustain. Energy Rev. 2019, 113, 109283. [Google Scholar] [CrossRef]
- Moreno, N.G.; Molina, M.C.; Gervasio, D.; Robles, J.F.P. Approaches to polymer electrolyte membrane fuel cells (PEMFCs) and their cost. Renew. Sustain. Energy Rev. 2015, 52, 897–906. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)—A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Alcaide, F.; Cabot, P.-L.; Brillas, E. Fuel cells for chemicals and energy cogeneration. J. Power Sources 2006, 153, 47–60. [Google Scholar] [CrossRef]
- Arshad, A.; Ali, H.M.; Habib, A.; Bashir, M.A.; Jabbal, M.; Yan, Y. Energy and exergy analysis of fuel cells: A review. Therm. Sci. Eng. Prog. 2019, 9, 308–321. [Google Scholar] [CrossRef]
- Wee, J.-H. Carbon dioxide emission reduction using molten carbonate fuel cell systems. Renew. Sustain. Energy Rev. 2014, 32, 178–191. [Google Scholar] [CrossRef]
- Rahman, S.; Masdar, M.S.; Rosli, M.; Majlan, E.; Husaini, T.; Kamarudin, S.; Daud, W. Overview biohydrogen technologies and application in fuel cell technology. Renew. Sustain. Energy Rev. 2016, 66, 137–162. [Google Scholar] [CrossRef]
- Jeon, D.H. Computational fluid dynamics simulation of anode-supported solid oxide fuel cells with implementing complete overpotential model. Energy 2019, 188, 116050. [Google Scholar] [CrossRef]
- Wang, H.-N.; Zhu, X.; Zhang, B.; Ye, D.; Chen, R.; Liao, Q.; Sui, P.-C.; Djilali, N. Two-phase computational modelling of a membraneless microfluidic fuel cell with a flow-through porous anode. J. Power Sources 2019, 420, 88–98. [Google Scholar] [CrossRef]
- Ly, H.; Birgersson, E.; Vynnycky, M. Computationally efficient multi-phase models for a proton exchange membrane fuel cell: Asymptotic reduction and thermal decoupling. Int. J. Hydrog. Energy 2011, 36, 14573–14589. [Google Scholar] [CrossRef]
- Vorobev, A.; Zikanov, O.; Shamim, T. A computational model of a PEM fuel cell with finite vapor absorption rate. J. Power Sources 2007, 166, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Birgersson, E.; Khor, S. Computationally-efficient hybrid strategy for mechanistic modeling of fuel cell stacks. J. Power Sources 2014, 247, 481–488. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Shabani, B. Proton exchange membrane fuel cells heat recovery opportunities for combined heating/cooling and power applications. Energy Convers. Manag. 2020, 204, 112328. [Google Scholar] [CrossRef]
- Amoatey, P.; Omidvarborna, H.; Baawain, M.S.; Al-Mamun, A. Emissions and exposure assessments of SOX, NOX, PM10/2.5 and trace metals from oil industries: A review study (2000–2018). Process. Saf. Environ. Prot. 2019, 123, 215–228. [Google Scholar] [CrossRef]
- Kamarudin, S.; Hashim, N. Materials, morphologies and structures of MEAs in DMFCs. Renew. Sustain. Energy Rev. 2012, 16, 2494–2515. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Akbari, E.; Buntat, Z.; Nikoukar, A.; Kheirandish, A.; Khaledian, M.; Afroozeh, A.; Khaledian, M. Sensor application in Direct Methanol Fuel Cells (DMFCs). Renew. Sustain. Energy Rev. 2016, 60, 1125–1139. [Google Scholar] [CrossRef]
- Deng, R.; Xia, Z.; Sun, R.; Wang, S.; Sun, G. Nanostructured ultrathin catalyst layer with ordered platinum nanotube arrays for polymer electrolyte membrane fuel cells. J. Energy Chem. 2020, 43, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Shahgaldi, S.; Ozden, A.; Alaefour, I.E.; Li, X.; Hamdullahpur, F. Effect of catalyst deposition on electrode structure, mass transport and performance of polymer electrolyte membrane fuel cells. Appl. Energy 2019, 255, 113802. [Google Scholar] [CrossRef]
- Star, A.G.; Wang, G.; Medina, S.; Pylypenko, S.; Neyerlin, K. Mass transport characterization of platinum group metal-free polymer electrolyte fuel cell electrodes using a differential cell with an integrated electrochemical sensor. J. Power Sources 2020, 450, 227655. [Google Scholar] [CrossRef]
- Lee, C.; Lee, J.; Zhao, B.; Fahy, K.; Lamanna, J.; Baltic, E.; Hussey, D.; Jacobson, D.; Schulz, V.; Bazylak, A. Temperature-dependent gas accumulation in polymer electrolyte membrane electrolyzer porous transport layers. J. Power Sources 2020, 446, 227312. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Singh, G.; Kim, K.S. Recent progress in the development of anode and cathode catalysts for direct methanol fuel cells. Nano Energy 2013, 2, 553–578. [Google Scholar] [CrossRef]
- Wang, J. System integration, durability and reliability of fuel cells: Challenges and solutions. Appl. Energy 2017, 189, 460–479. [Google Scholar] [CrossRef]
- Brouzgou, A.; Song, S.; Tsiakarasbcd, P. Low and non-platinum electrocatalysts for PEMFCs: Current status, challenges and prospects. Appl. Catal. B Environ. 2012, 127, 371–388. [Google Scholar] [CrossRef]
- Kulikovsky, A. Direct methanol–hydrogen fuel cell: The mechanism of functioning. Electrochem. Commun. 2008, 10, 1415–1418. [Google Scholar] [CrossRef]
- Pramanik, H.; Basu, S. Modeling and experimental validation of overpotentials of a direct ethanol fuel cell. Chem. Eng. Process. Process. Intensif. 2010, 49, 635–642. [Google Scholar] [CrossRef]
- Jeng, K.; Chen, C. Modeling and simulation of a direct methanol fuel cell anode. J. Power Sources 2002, 112, 367–375. [Google Scholar] [CrossRef]
- Kulikovsky, A. Analytical model of the anode side of DMFC: The effect of non-Tafel kinetics on cell performance. Electrochem. Commun. 2003, 5, 530–538. [Google Scholar] [CrossRef]
- Rosenthal, N.S.; Vilekar, S.A.; Datta, R. A comprehensive yet comprehensible analytical model for the direct methanol fuel cell. J. Power Sources 2012, 206, 129–143. [Google Scholar] [CrossRef]
- Kulikovsky, A. Comment on “A one dimensional model of a methanol fuel cell anode” [K. Scott, P. Argyropoulos, J. Power Sources 137 (2004) 228]. J. Power Sources 2005, 148, 54. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T. Mathematical modeling of a passive-feed DMFC with heat transfer effect. J. Power Sources 2005, 152, 122–130. [Google Scholar] [CrossRef]
- Theodosiou, P.; Greenman, J.; Ieropoulos, I. Towards monolithically printed Mfcs: Development of a 3d-printable membrane electrode assembly (mea). Int. J. Hydrog. Energy 2019, 44, 4450–4462. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, W.; Su, H.; Leung, P.; Xing, L.; Xu, L.; Yang, C.; Xu, Q. Improving cell performance and alleviating performance degradation by constructing a novel structure of membrane electrode assembly (MEA) of DMFCs. Int. J. Hydrog. Energy 2019, 44, 32231–32239. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, Y.; Zuo, P.-J.; Wang, X.-P.; Yin, G.-P. Durability studies of unsupported Pt cathodic catalyst with working time of direct methanol fuel cells. J. Power Sources 2008, 185, 1066–1072. [Google Scholar] [CrossRef]
- Jiang, R.; Rong, C.; Chu, D. Fuel Crossover and Energy Conversion in Lifetime Operation of Direct Methanol Fuel Cells. J. Electrochem. Soc. 2007, 154, B13. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Z.; Zhao, X.; Xin, Q.; Sun, G.; Yi, B. Studies on performance degradation of a direct methanol fuel cell (DMFC) in life test. Phys. Chem. Chem. Phys. 2004, 6, 134. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef] [Green Version]
- Iulianelli, A.; Basile, A. Sulfonated PEEK-based polymers in PEMFC and DMFC applications: A review. Int. J. Hydrog. Energy 2012, 37, 15241–15255. [Google Scholar] [CrossRef]
- Slater, J.; Chronopoulos, T.; Panesar, R.; Fitzgerald, F.; Garcia, M. Review and techno-economic assessment of fuel cell technologies with CO2 capture. Int. J. Greenh. Gas Control 2019, 91, 102818. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. End of life of fuel cells and hydrogen products: From technologies to strategies. Int. J. Hydrog. Energy 2019, 44, 20965–20977. [Google Scholar] [CrossRef]
- Xing, L.; Shi, W.; Su, H.; Xu, Q.; Das, P.; Mao, B.; Scott, K. Membrane electrode assemblies for PEM fuel cells: A review of functional graded design and optimization. Energy 2019, 177, 445–464. [Google Scholar] [CrossRef]
- Abdel-Rehim, A.A. The influence of electromagnetic field on the performance and operation of a PEM fuel cell stack subjected to a relatively low electromagnetic field intensity. Energy Convers. Manag. 2019, 198, 111906. [Google Scholar] [CrossRef]
- Niroumand, A.M.; Homayouni, H.; Goransson, G.; Olfert, M.; Eikerling, M. In-situ diagnostic tools for hydrogen transfer leak characterization in PEM fuel cell stacks part III: Manufacturing applications. J. Power Sources 2020, 448, 227359. [Google Scholar] [CrossRef]
- Haragirimana, A.; Ingabire, P.B.; Zhu, Y.; Lu, Y.; Li, N.; Hu, Z.; Chen, S. Four-polymer blend proton exchange membranes derived from sulfonated poly(aryl ether sulfone)s with various sulfonation degrees for application in fuel cells. J. Membr. Sci. 2019, 583, 209–219. [Google Scholar] [CrossRef]
- Pasini, D.; Nitti, A. Free radical cyclopolymerization: A tool towards sequence control in functional polymers. Eur. Polym. J. 2020, 122, 109378. [Google Scholar] [CrossRef]
- Simya, O.K.; Radhakrishnan, P.; Ashok, A. Engineered Nanomaterials for Energy Applications. In Handbook of Nanomaterials for Industrial Applications; Elsevier BV: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Peng, F.; Ren, L.; Zhao, Y.; Li, L. Hybrid dynamic modeling-based membrane hydration analysis for the commercial high-power integrated PEMFC systems considering water transport equivalent. Energy Convers. Manag. 2020, 205, 112385. [Google Scholar] [CrossRef]
- Nanadegani, F.S.; Lay, E.N.; Iranzo, A.; Salva, J.A.; Sundén, B. On neural network modeling to maximize the power output of PEMFCs. Electrochim. Acta 2020, 348, 136345. [Google Scholar] [CrossRef]
- Yang, Z.; Jiao, K.; Liu, Z.; Yin, Y.; Du, Q. Investigation of performance heterogeneity of PEMFC stack based on 1+1D and flow distribution models. Energy Convers. Manag. 2020, 207, 112502. [Google Scholar] [CrossRef]
- Chugh, S.; Chaudhari, C.; Sonkar, K.; Sharma, A.; Kapur, G.; Ramakumar, S. Experimental and modelling studies of low temperature PEMFC performance. Int. J. Hydrog. Energy 2020, 45, 8866–8874. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, D.; Yi, P.; Lan, S.; Peng, L. An integrated model of the water transport in nonuniform compressed gas diffusion layers for PEMFC. Int. J. Hydrog. Energy 2019, 44, 13777–13785. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Mérida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Lv, Z.; Zhao, T.; Li, P.; Sun, Y.; Wang, J. Sulfonated Ti3C2Tx to construct proton transfer pathways in polymer electrolyte membrane for enhanced conduction. Solid State Ion. 2017, 310, 100–111. [Google Scholar] [CrossRef]
- Yu, L.; Yue, B.; Yan, L.; Zhao, H.; Zhang, J. Proton conducting composite membranes based on sulfonated polysulfone and polysulfone-g-(phosphonated polystyrene) via controlled atom-transfer radical polymerization for fuel cell applications. Solid State Ion. 2019, 338, 103–112. [Google Scholar] [CrossRef]
- Delemotte, L.; Van Keulen, S.; Roethlisberger, U.; Gianti, E.; Carnevale, V.; Klein, M.L. Does Proton Conduction in the Voltage-Gated Proton Channel hH V 1 Involve Grotthus Hopping via Acidic Residues? Biophys. J. 2017, 112, 163a–164a. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, V.; Son, T.Y.; Kim, H.J.; Nam, S.Y. A facile approach to fabricate poly(2,6-dimethyl-1,4-phenylene oxide) based anion exchange membranes with extended alkaline stability and ion conductivity for fuel cell applications. J. Membr. Sci. 2019, 591, 117314. [Google Scholar] [CrossRef]
- Yadav, V.; Rajput, A.; Sharma, P.P.; Jha, P.K.; Kulshrestha, V. Polyetherimide based anion exchange membranes for alkaline fuel cell: Better ion transport properties and stability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124348. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Liu, S.; Li, H.; Zhu, K. Optimization of reactants relative humidity for high performance of polymer electrolyte membrane fuel cells with co-flow and counter-flow configurations. Energy Convers. Manag. 2020, 205, 112369. [Google Scholar] [CrossRef]
- Damo, U.M.; Ferrari, M.; Turan, A.; Massardo, A. Solid oxide fuel cell hybrid system: A detailed review of an environmentally clean and efficient source of energy. Energy 2019, 168, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Raza, R.; Zhu, B.; Rafique, A.; Naqvi, M.R.; Lund, P. Functional ceria-based nanocomposites for advanced low-temperature (300–600 °C) solid oxide fuel cell: A comprehensive review. Mater. Today Energy 2020, 15, 100373. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Wang, X.; Bi, L. Sintering aids for proton-conducting oxides—A double-edged sword? A mini review. Electrochem. Commun. 2020, 112, 106672. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Ma, J.; Liu, W.; Wang, X.; Fronzi, M.; Bi, L. Impressive performance of proton-conducting solid oxide fuel cells using a first-generation cathode with tailored cations. J. Mater. Chem. A 2019, 7, 18792–18798. [Google Scholar] [CrossRef]
- Tarutina, L.R.; Vdovin, G.K.; Lyagaeva, J.G.; Medvedev, D.A. BaCe0.7–xZr0.2Y0.1FexO3–δ derived from proton-conducting electrolytes: A way of designing chemically compatible cathodes for solid oxide fuel cells. J. Alloys Compd. 2020, 831, 154895. [Google Scholar] [CrossRef]
- Mojaver, P.; Chitsaz, A.; Sadeghi, M.; Khalilarya, S. Comprehensive comparison of SOFCs with proton-conducting electrolyte and oxygen ion-conducting electrolyte: Thermoeconomic analysis and multi-objective optimization. Energy Convers. Manag. 2020, 205, 112455. [Google Scholar] [CrossRef]
- Xu, X.; Bi, L. Proton-conducting electrolyte materials. Intermed. Temp. Solid Oxide Fuel Cells 2020, 81–111. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Sadeghzadeh, M.; Rahimi, A.; Pouriman, H. Technical performance analysis of a combined cooling heating and power (CCHP) system based on solid oxide fuel cell (SOFC) technology—A building application. Energy Convers. Manag. 2019, 198, 111767. [Google Scholar] [CrossRef]
- Winkler, W.; Lorenz, H. Design studies of mobile applications with SOFC–heat engine modules. J. Power Sources 2002, 106, 338–343. [Google Scholar] [CrossRef]
- Jurado, F. Modeling SOFC plants on the distribution system using identification algorithms. J. Power Sources 2004, 129, 205–215. [Google Scholar] [CrossRef]
- Jurado, F.; Valverde, M.; Cano, A. Effect of a SOFC plant on distribution system stability. J. Power Sources 2004, 129, 170–179. [Google Scholar] [CrossRef]
- Van Herle, J.; Wang, L.; Leuenberger, S.; Favrat, D. Energy balance model of a SOFC cogenerator operated with biogas. J. Power Sources 2003, 118, 375–383. [Google Scholar] [CrossRef]
- Petruzzi, L.; Cocchi, S.; Fineschi, F. A global thermo-electrochemical model for SOFC systems design and engineering. J. Power Sources 2003, 118, 96–107. [Google Scholar] [CrossRef]
- Padulles, J.; Ault, G.; McDonald, J. An integrated SOFC plant dynamic model for power systems simulation. J. Power Sources 2000, 86, 495–500. [Google Scholar] [CrossRef]
- Walters, K.M.; Dean, A.; Zhu, H.; Kee, R.J. Homogeneous kinetics and equilibrium predictions of coking propensity in the anode channels of direct oxidation solid-oxide fuel cells using dry natural gas. J. Power Sources 2003, 123, 182–189. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W.; Yang, C.; Gao, L.; Thangavel, S.; Wang, L.; He, Z.; Cai, W.; Wang, A. Computational and experimental analysis of organic degradation positively regulated by bioelectrochemistry in an anaerobic bioreactor system. Water Res. 2017, 125, 170–179. [Google Scholar] [CrossRef]
- Sammes, N.; Galloway, K.; Serincan, M.; Suzuki, T.; Yamaguchi, T.; Awano, M.; Colella, W. Solid Oxide Fuel Cells. In Handbook of Climate Change Mitigation; Springer Science and Business Media LLC: New York, NY, USA, 2012; pp. 1703–1727. [Google Scholar]
- Virkar, A.V.; Chen, J.; Tanner, C.W.; Kim, J.-W. The role of electrode microstructure on activation and concentration polarizations in solid oxide fuel cells. Solid State Ion. 2000, 131, 189–198. [Google Scholar] [CrossRef]
- Fukunaga, H.; Ihara, M.; Sakaki, K.; Yamada, K. The relationship between overpotential and the three phase boundary length. Solid State Ion. 1996, 86, 1179–1185. [Google Scholar] [CrossRef]
- Iwata, M.; Hikosaka, T.; Morita, M.; Iwanari, T.; Ito, K.; Onda, K.; Esaki, Y.; Sakaki, Y.; Nagata, S. Performance analysis of planar-type unit SOFC considering current and temperature distributions. Solid State Ion. 2000, 132, 297–308. [Google Scholar] [CrossRef]
- Haynes, C.; Wepfer, W.J. ‘Design for power’ of a commercial grade tubular solid oxide fuel cell. Energy Convers. Manag. 2000, 41, 1123–1139. [Google Scholar] [CrossRef]
- Larrain, D.; Van Herle, J.; Wang, L.; Favrat, D. Generalized model of planar SOFC repeat element for design optimization. J. Power Sources 2004, 131, 304–312. [Google Scholar] [CrossRef]
- Khaleel, M.; Lin, Z.; Singh, P.; Surdoval, W.; Collin, D. A finite element analysis modeling tool for solid oxide fuel cell development: Coupled electrochemistry, thermal and flow analysis in MARC®. J. Power Sources 2004, 130, 136–148. [Google Scholar] [CrossRef]
- Costamagna, P.; De Giorgi, A.; Moser, G.; Pellaco, L.; Trucco, A. Data-driven fault diagnosis in SOFC-based power plants under off-design operating conditions. Int. J. Hydrog. Energy 2019, 44, 29002–29006. [Google Scholar] [CrossRef]
- Boersma, R.; Sammes, N. Computational analysis of the gas-flow distribution in solid oxide fuel cell stacks. J. Power Sources 1996, 63, 215–219. [Google Scholar] [CrossRef]
- Boersma, R.; Sammes, N. Distribution of gas flow in internally manifolded solid oxide fuel-cell stacks. J. Power Sources 1997, 66, 41–45. [Google Scholar] [CrossRef]
- Dotelli, G.; Sora, I.N.; Schmid, C.; Mari, C. Composite materials as electrolytes for solid oxide fuel cells: Simulation of microstructure and electrical properties. Solid State Ion. 2002, 152, 509–515. [Google Scholar] [CrossRef]
- Rong, G.; Jin, M.; Shuai, L.; Guo, X. Centroidal Voronoi tessellation in universal covering space of manifold surfaces. Comput. Aided Geom. Des. 2011, 28, 475–496. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.; Taama, W.; Cruickshank, J. Performance and modelling of a direct methanol solid polymer electrolyte fuel cell. J. Power Sources 1997, 65, 159–171. [Google Scholar] [CrossRef]
- Siegel, C. Review of computational heat and mass transfer modeling in polymer-electrolyte-membrane (PEM) fuel cells. Energy 2008, 33, 1331–1352. [Google Scholar] [CrossRef]
- Radenahmad, N.; Azad, A.T.; Saghir, M.; Taweekun, J.; Abu Bakar, M.S.; Reza, S.; Azad, A.K. A review on biomass derived syngas for SOFC based combined heat and power application. Renew. Sustain. Energy Rev. 2020, 119, 109560. [Google Scholar] [CrossRef]
- Saied, M.; Ahmed, K.; Nemat-Alla, M.; Ahmed, M.; El-Sebaie, M. Performance study of solid oxide fuel cell with various flow field designs: Numerical study. Int. J. Hydrog. Energy 2018, 43, 20931–20946. [Google Scholar] [CrossRef]







| Benefits | The consistency of the size and air flow in SOFC stack size is maintained. Pressure value is maintained along with the pressured existing SOFC stacks. Turbine inlet temperature values close to stack discharge conditions. Available air temperature values near to SOFC cathode inlet. Promising electrical integration at continuous current level. |
| Limitations | Commercial microturbines not specially premeditated for SOFC. Substantial impact of ambient temperature value. Plant exhaust flow temperature unable to decrease less than 200 to 250 °C. The controllability of dynamic issues. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. https://doi.org/10.3390/membranes10050099
Sazali N, Wan Salleh WN, Jamaludin AS, Mhd Razali MN. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes. 2020; 10(5):99. https://doi.org/10.3390/membranes10050099
Chicago/Turabian StyleSazali, Norazlianie, Wan Norharyati Wan Salleh, Ahmad Shahir Jamaludin, and Mohd Nizar Mhd Razali. 2020. "New Perspectives on Fuel Cell Technology: A Brief Review" Membranes 10, no. 5: 99. https://doi.org/10.3390/membranes10050099