Removal of Dye from a Leather Tanning Factory by Flat-Sheet Blend Ultrafiltration (UF) Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
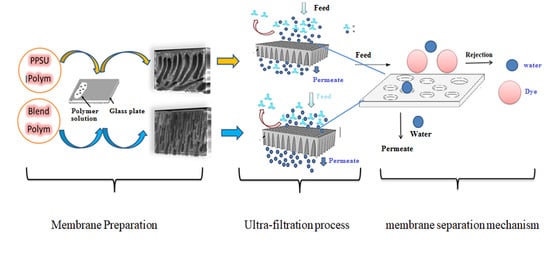
2.2. Membrane Preparation
2.3. Membrane Surface Characteristics
2.3.1. Contact Angle (CA) of the Membranes
2.3.2. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Atomic Force Microscopy
2.3.5. Porosity Measurement
2.3.6. Mechanical Stability Measurement
2.3.7. Membrane Performance
2.4. Solubility Parameter Difference (∆δ)
3. Results and Discussion
3.1. Influence of PES on PPSU Membrane Morphology
3.2. Fourier-Transform Infrared (FTIR) Spectroscopy
3.3. Effect of PES on the PPSU Membrane Surface Roughness
3.4. PPSU-PES Membranes Hydrophilicity
3.5. Effects of PES on Membrane Thickness and Porosity
3.6. Performance of the PPSU-PES Membrane
3.7. Effect of PES on Mechanical Properties
3.8. Dye removal Efficiency of the PPSU-PES Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Zhong, Z.; Ma, R.; Zhang, W.; Li, J. Development of High-Antifouling PPSU Ultrafiltration Fiber by Using Compound Additives: Preparation, Morphologies, and Filtration Resistant Properties. Fibers 2016, 6, 35. [Google Scholar]
- Kamar, F.H.; Craciun, M.E.; Nechifor, A.C. Heavy metals: Sources, health effects, environmental effects, removal methods and natura l adsorbent material as low-cost adsorbent: Short review. Int. J. Sci. Eng. Technol. Res. 2014, 3, 2974–2979. [Google Scholar]
- Koyuncu, I. Reactive dye removal in dye/salt mixtures by nanofiltration fibers containing vinylsulphone dyes: Effects of feed concentration and cross flow velocity. Desalination 2002, 143, 243–253. [Google Scholar] [CrossRef]
- Majewska-Nowak, K. Ultrafiltration of dye solutions in the presence of cationic and anionic surfactants. Environ. Prot. Eng. 2009, 35, 111–121. [Google Scholar]
- Alventosa-deLara, E.; Barredo-Damas, S.; Alcaina-Miranda, M.I.; Iborra-Clar, M.I. Ultrafiltration technology with a ceramic fiber for reactive dye removal: Optimization of fiber performance. J. Hazard. Mater. 2012, 209, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Moideen, K.I.; Isloor, A.M.; Ismail, A.F.; Obaid, A.; Fun, H.K. Fabrication and characterization of new PSF/PPSU UF blend fiber for heavy metal rejection. Desalin. Water Treat. 2016, 57, 19810–19819. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Ali, J.M.; Abbas, A.A.; Rashed, A.; Bruggen, B.V.D.; Balta, S. Enhancement of poly (phenyl sulfone) fibers with ZnO nanoparticles. Desalin. Water Treat. 2013, 51, 6070–6081. [Google Scholar] [CrossRef]
- Al-Ani, D.M.; Al-Ani, F.H.; Alsalhy, Q.F.; Ibrahim, S.S. Preparation and characterization of ultrafiltration membranes from PPSU-PES polymer blend for dyes removal. Chem. Eng. Comun. 2019, 1–19. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Fiber distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Rashid, K.T.; Noori, W.A.; Figoli, A.; Simone, S.; Drioli, E. Poly (vinyl chloride) hollow fibers fibers for ultrafiltration applications: Effects of internal coagulant composition. J. Appl. Polymer Sci. 2012, 124, 2087–2099. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Mohammed, A.A.; Ahmed, S.H.; Rashid, K.T.; AlSaadi, M.A. Estimation of nanofiltration fiber transport parameters for cobalt ions removal from aqueous solutions. Desalin. Water Treat. 2018, 108, 235–245. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H. Polymer Handbook, 3rd ed.; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Alsalhy, Q.F.; Rashid, K.T.; Ibrahim, S.S.; Ghanim, A.H.; Van der Bruggen, B.; Luis, P.; Zablouk, M. Poly (vinylidene fluoride-co-hexafluoropropylene)(PVDF-co-HFP) hollow fiber fibers prepared from PVDF-co-HFP/PEG-600Mw/DMAC solution for fiber distillation. J. Appl. Polym. Sci. 2013, 129, 3304–3313. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, K.H. Effect of PEG additive on fiber formation by phase inversion. J. Membr. Sci. 1998, 138, 153–163. [Google Scholar] [CrossRef]
- Pakizeh, M.; Mansoori, S.A.A.; Pourafshari Chenar, M.; Namvar-Mahboub, M. Modification of PSf membrane nanostructure using different fabrication parameters and investigation of the CO2 separation properties of PDMS-coated PSf composite membranes. Braz. J. Chem. Eng. 2013, 30, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Qin, H.; Shi, W.; Sun, S.; Zhao, C. Surface modification of poly (ether sulfone) fiber with a synthesized negatively charged copolymer. Langmuir 2014, 30, 13622–13630. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Mukhtar, H.B.; Shariff, A.M. Research Article FTIR Study of Enhanced Polymeric Blend Fiber with Amines. Res. J. Appl. Sci. Eng. Technol. 2014, 7, 1811–1820. [Google Scholar] [CrossRef]
- Golpour, M.; Pakizeh, M. Development of a new nanofiltration fiber for removal of kinetic hydrate inhibitor from water. Sep. Purif. Technol. 2017, 183, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Ma, J.; Shi, X.; Ren, Z. Effect of TiO2 nanoparticle size on the performance of PVDF fiber. Appl. Surf. Sci. 2006, 253, 2003–2010. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES fibers: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Hamid, N.A.A.; Ismail, A.F.; Matsuura, T.; Zularisam, A.W.; Lau, W.J.; Yuliwati, E.; Abdullah, M.S. Morphological and separation performance study of polysulfone/titanium dioxide (PSF/TiO2) ultrafiltration fibers for humic acid removal. Desalination 2011, 273, 85–92. [Google Scholar] [CrossRef]
- Hong, J.; He, Y. Effects of nano sized zinc oxide on the performance of PVDF microfiltration fibers. Desalination 2012, 302, 71–79. [Google Scholar] [CrossRef]
- Rajesh, S.; Maheswari, P.; Senthilkumar, S.; Jayalakshmi, A.; Mohan, D. Preparation and characterisation of poly (amide-imide) incorporated cellulose acetate membranes for polymer enhanced ultrafiltration of metal ions. Chem. Eng. J. 2011, 171, 33–44. [Google Scholar] [CrossRef]
- Hong, J.; He, Y. Polyvinylidene fluoride ultrafiltration fiber blended with nano-ZnO particle for photo-catalysis self-cleaning. Desalination 2014, 332, 67–75. [Google Scholar] [CrossRef]
- Li, J.F.; Xu, Z.L.; Yang, H. Microporous polyethersulfone fibers prepared under the combined precipitation conditions with non-solvent additives. Polym. Adv. Technol. 2008, 19, 251–257. [Google Scholar] [CrossRef]
- Luo, L.; Chung, T.S.; Weber, M.; Staudt, C.; Maletzko, C. Molecular interaction between acidic sPPSU and basic HPEI polymers and its effects on fiber formation for ultrafiltration. J. Membrane Sci. 2017, 524, 33–42. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Mehdipour-Ataei, S. Synthesis of a novel poly (amide-imide)(PAI) and preparation and characterization of PAI blended polyethersulfone (PES) fibers. J. Membrane Sci. 2008, 311, 349–359. [Google Scholar] [CrossRef]












| Membranes | Casting Solution Compositions (wt.%) | |
|---|---|---|
| PPSU | PES | |
| P1 | 20 | 0 |
| P2 | 19 | 1 |
| P3 | 18 | 2 |
| P4 | 17 | 3 |
| P5 | 16 | 4 |
| Component | ᵟd ((MPa)0.5) | ᵟp ((MPa)0.5) | ᵟh ((MPa)0.5) | ∆ᵟs-PPSU | ∆ᵟs-mix | ∆ᵟs-water |
|---|---|---|---|---|---|---|
| NMP | 18.00 | 12.30 | 7.20 | - | - | - |
| P2 | 18.11 | 11.11 | 7.23 | - | 1.19 | - |
| P3 | 18.10 | 11.15 | 7.24 | - | 1.14 | - |
| P4 | 18.09 | 11.22 | 7.24 | - | 1.07 | - |
| P5 | 18.08 | 11.25 | 7.24 | - | 1.05 | - |
| PPSU | 18.70 | 05.00 | 7.40 | 7.33 | - | - |
| PES | 17.60 | 10.40 | 7.80 | - | - | - |
| WATER | 15.50 | 16.00 | 42.40 | - | - | 35.48 |
| Membrane | Ra (nm) | Mean Pore Size (nm) | ||
|---|---|---|---|---|
| Top Surface | Bottom Surface | Top Surface | Bottom Surface | |
| P1 | 12 | 8 | 60.55 | 56.26 |
| P2 | 16 | 362 | 87.21 | 83.84 |
| P3 | 245 | 402 | 113.28 | 84.12 |
| P4 | 964 | 189 | 83.4 | 62.96 |
| P5 | 255 | 169 | 120.4 | 112.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghadhban, M.Y.; Majdi, H.S.; Rashid, K.T.; Alsalhy, Q.F.; Lakshmi, D.S.; Salih, I.K.; Figoli, A. Removal of Dye from a Leather Tanning Factory by Flat-Sheet Blend Ultrafiltration (UF) Membrane. Membranes 2020, 10, 47. https://doi.org/10.3390/membranes10030047
Ghadhban MY, Majdi HS, Rashid KT, Alsalhy QF, Lakshmi DS, Salih IK, Figoli A. Removal of Dye from a Leather Tanning Factory by Flat-Sheet Blend Ultrafiltration (UF) Membrane. Membranes. 2020; 10(3):47. https://doi.org/10.3390/membranes10030047
Chicago/Turabian StyleGhadhban, Maryam Y., Hasan Shaker Majdi, Khalid T. Rashid, Qusay F. Alsalhy, D. Shanthana Lakshmi, Issam K. Salih, and Alberto Figoli. 2020. "Removal of Dye from a Leather Tanning Factory by Flat-Sheet Blend Ultrafiltration (UF) Membrane" Membranes 10, no. 3: 47. https://doi.org/10.3390/membranes10030047