Theoretical and Experimental Optimization of the Graft Density of Functionalized Anti-Biofouling Surfaces by Cationic Brushes
Abstract
:1. Introduction
2. Theoretical Calculations
3. Experimental Section
3.1. Materials
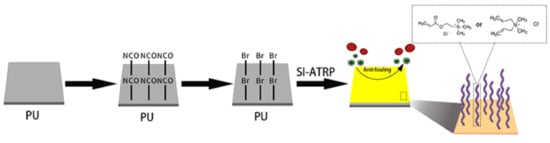
3.2. Preparation of PU Films
3.3. Immobilization of Initiator on PU Films
3.4. SI-ATRP from PU Films
3.5. Characterization
3.6. Protein Adsorption of PU
3.7. Hemolysis Assays of PU
3.8. Anticoagulant Measurements of PU In Vitro
3.9. E. coli Adhesion to PU
4. Results and Discussion
4.1. The Intermolecular Hydrogen Bonds
4.2. PU Grafting Density
4.3. Characterization of Functionalized PU Samples
4.4. Protein Adsorption
4.5. Hemocompatibility In Vitro
4.6. E. coli Adhesion
4.7. Zeta Potential
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rickard, C.M.; Nicole, M.; Joan, W.; Runnegar, N.; Larsen, E.; McGrail, M.R.; Fullerton, F.; Bettington, E.; Whitty, J.A.; Choudhury, M.A.; et al. Dressings and securements for the prevention of peripheral intravenous catheter failure in adults (SAVE): A pragmatic, randomised controlled, superiority trial. Lancet 2018, 392, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Ariza, H.; Ella, J. Update on infection control practices in cancer hospitals. CA Cancer J. Clin. 2018, 68, 340–355. [Google Scholar] [CrossRef] [Green Version]
- Laura, L. Antibiotic locks for the treatment of catheter-related blood stream infection: Still more hope than data. Semin. Dial. 2019, 32, 402–405. [Google Scholar]
- Liang, W.; Wei, F.; Yang, C. GDF-15 is associated with thrombus burden in patients with deep venous thrombosis. Thromb. Res. 2020, 187, 148–153. [Google Scholar] [CrossRef]
- Keum, H.; Kim, J.Y.; Yu, B.; Yu, S.J.; Kim, J.; Jeon, H.; Lee, D.Y.; Im, S.G.; Jon, S. Prevention of bacterial colonization on catheters by a one-step coating process involving an antibiofouling Polymer in Water. ACS Appl. Mater. Interfaces 2017, 9, 19736–19745. [Google Scholar] [CrossRef]
- Riool, M.; de Breij, A.; Drijfhout, J.W.; Nibbering, P.H.; Zaat, S.A. Antimicrobial peptides in biomedical device manufacturing. Front. Chem. 2017, 5, 63. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Soveri, I.; Hilborn, J.; Fellström, B.; Nilsson, B. Cardiovascular disease in haemodialysis: Role of the intravascular innate immune system. Nat. Rev. Nephrol. 2017, 13, 285–296. [Google Scholar] [CrossRef]
- Murray, J.; Precious, E.; Alikhan, R. Catheter-related thrombosis in cancer patients. Br. J. Haematol. 2013, 162, 748–757. [Google Scholar] [CrossRef]
- Yamada, S.; Motozuka, S.; Tagaya, M. Synthesis of nanostructured silica/hydroxyapatite hybrid particles containing amphiphilic triblock copolymer for effectively controlling hydration layer structures with cytocompatibility. J. Mater. Chem. B 2020, 8, 1524–1537. [Google Scholar] [CrossRef]
- Adamczyk, Z. Protein adsorption: A quest for a universal mechanism. Curr. Opin. Colloid Interface Sci. 2019, 41, 50–65. [Google Scholar] [CrossRef]
- Stepniewski, M.; Pasenkiewicz-Gierula, M.; Ro, G.T.; Danne, R.; Orlowski, A.; Karttunen, M.; Urtti, A.; Yliperttula, M.; Vuorimaa, E.; Bunker, A. Study of PEGylated lipid layers as a model for PEGylated liposome surfaces: Molecular dynamics simulation and Langmuir monolayer studies. Langmuir 2011, 27, 7788–7798. [Google Scholar] [CrossRef] [PubMed]
- Cormack, A.N.; Lewis, R.J.; Goldstein, A.H. Computer simulation of protein adsorption to a material surface in aqueous solution: Biomaterials modeling of a ternary system. J. Phys. Chem. B 2004, 108, 20408–20418. [Google Scholar] [CrossRef]
- Panos, M.; Sen, T.Z.; Ahunbay, M.G. Molecular simulation of fibronectin adsorption onto polyurethane surfaces. Langmuir 2012, 28, 12619–12628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, X.; Wang, Z.; Huang, X.; Ge, Z.; Hu, B. Influence of structured water layers on protein adsorption process: A case study of cytochrome c and carbon nanotube interactions and its implications. J. Phys. Chem. B 2020, 124, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, X.; Zhang, K.; Wu, M.Y.; Wu, Q.Y.; Liu, J.Y.; Yang, J.J.; Zhang, J.N. Nano-hydroxyapatite particle brushes via direct initiator tethering and surface-initiated atom transfer radical polymerization for dual responsive Pickering emulsion. Langmuir 2020, 36, 1192–1200. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, M.; Ding, K. Cell membrane mimetic PVDF microfiltration membrane with enhanced antifouling and separation performance for oil/water mixtures. J. Mater. Chem. A 2018, 6, 3231–3241. [Google Scholar] [CrossRef]
- Debayle, M.; Balloul, E.; Dembele, F.; Xu, X.; Hanafi, M.; Ribot, F.; Monzel, C.; Coppey, M.; Fragola, A.; Dahan, M.; et al. Zwitterionic polymer ligands: An ideal surface coating to totally suppress protein-nanoparticle corona formation. Biomaterials 2019, 219, 119357. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yin, C.; Wang, Y. Simultaneous zwitterionization and selective swelling-induced pore generation of block copolymers for antifouling ultrafiltration membranes. J. Membr. Sci. 2020, 599, 117833. [Google Scholar] [CrossRef]
- Pullanchery, S.; Yang, T.; Cremer, P.S. Introduction of positive charges into zwitterionic phospholipid monolayers disrupts water structure whereas negative charges enhances it. J. Phys. Chem. B 2018, 122, 12260–12270. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ren, B. Fundamentals and applications of zwitterionic antifouling polymers. J. Phys. D Appl. Phys. 2019, 52, 403001. [Google Scholar] [CrossRef]
- Tsai, M.C.; Hung, K.C.; Hung, S.C.; Hsu, S.H. Evaluation of biodegradable elastic scaffolds made of anionic polyurethane for cartilage tissue engineering. Colloids Surf. B Biointerfaces 2015, 125, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chiao, M. Anti-fouling coatings of poly (dimethylsiloxane) devices for biological and biomedical applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasia, W.; Lei, C.; Cao, Y.; Ye, Q.; He, Y.; Xu, C. Enhanced eradication of bacterial biofilms with DNase I-loaded silver-doped mesoporous silica nanoparticles. Nanoscale 2020, 12, 2328–2332. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Faria, A.F.; Ma, J.; Elimelech, M. Mitigation of Biofilm Development on Thin-Film Composite Membranes Functionalized with Zwitterionic Polymers and Silver Nanoparticles. Environ. Sci. Technol. 2017, 51, 182–191. [Google Scholar] [CrossRef]
- He, Y.; Wan, X.; Xiao, K.; Lin, W.; Li, J.; Li, Z.; Luo, F.; Tan, H.; Li, J.; Fu, Q. Anti-biofilm surfaces from mixed dopamine modified polymer brushes: Synergistic role of cationic and zwitterionic chains to resist staphylococcus aureus. Biomater. Sci. 2019, 7, 5369–5382. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Yuan, J. Design of hemocompatible and antifouling PET sheets with synergistic zwitterionic surfaces. J. Colloid Interface Sci. 2016, 480, 205–217. [Google Scholar] [CrossRef]
- Jin, X.; Yuan, J.; Shen, J. Zwitterionic polymer brushes via dopamine-initiated ATRP from PET sheets for improving hemocompatible and antifouling properties. Colloids Surf. B Biointerfaces 2016, 145, 275–284. [Google Scholar] [CrossRef]
- Robinson, D.A.; Griffith, R.W.; Shechtman, D.; Evans, R.B.; Conzemius, M.G. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010, 6, 1869–1877. [Google Scholar] [CrossRef]
- Li, P.; Poon, Y.F.; Li, W.; Zhu, H.Y.; Yeap, S.H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.W.; et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2010, 10, 149–156. [Google Scholar] [CrossRef]
- Gao, D.; Feng, J.; Ma, J. Synthesis of cationic binder through surfactant-free emulsion polymerization for textile pigment applications. Prog. Org. Coat. 2014, 77, 1834–1840. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, W.; Xu, J.; Xue, W.; Liu, Z. Evaluation of N-phosphonium chitosan as a novel vaccine carrier for intramuscular immunization. J. Biomater. Appl. 2017, 32, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Yao, G.; Sun, Z.; Wang, B.; Yu, C.; Zheng, S. Fabrication of a novel antibacterial TPU nanofiber membrane containing Cu-loaded zeolite and its antibacterial activity toward Escherichia coli. J. Mater. Sci. 2019, 54, 11682–11693. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, Z.; Liang, L.; Lan, K.; Zheng, Q.; Wang, Y.; Guo, Y.; Zhu, K.; Mehmood, R.; Wang, B. A facile heparin/carboxymethyl chitosan coating mediated by polydopamine on implants for hemocompatibility and antibacterial properties. Appl. Surf. Sci. 2020, 528, 146539. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMm: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMm general force field: A force field for drug-like molecules compatible with the CHARMm all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Jin, J.; Park, S.; Kim, J.Y.; Lee, M.J.; Sun, H.; Kwon, J.S.; Lee, H.; Choi, S.H.; Hong, J. Quantitative interpretation of hydration dynamics enabled the fabrication of a zwitterionic antifouling surface. ACS Appl. Mater. Interfaces 2020, 12, 7951–7965. [Google Scholar] [CrossRef]
- Walke, C.D.; Chan, W.C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Liu, P.; Huang, T.; Liu, P.; Shi, S.; Chen, Q.; Li, L.; Shen, J. Zwitterionic modification of polyurethane membranes for enhancing the anti-fouling property. J. Colloid Interface Sci. 2016, 363, 483–489. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y.; Lynch, D.; Young, M.; Li, S.; Cong, H.; Xu, F.J.; Cheng, G. Zwitterionic Polyurethanes with Tunable Surface and Bulk Properties. ACS Appl. Mater. Interfaces 2018, 10, 37609–37617. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, Y.; Wang, A.; Ding, X.; Li, Y.; Duan, S.; Ding, X.; Xu, F.J. Antimicrobial Peptide-Conjugated Hierarchical Antifouling Polymer Brushes for Functionalized Catheter Surfaces. Biomacromolecules 2019, 11, 4171–4179. [Google Scholar] [CrossRef]
- Shitole, A.A.; Raut, P.W.; Khandwekar, A. Design and engineering ofpolyvinyl alcohol based biomimetic hydrogels for wound healing and repair. J. Polym. Res. 2019, 26, 201. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Feng, F.; Wang, D.; Lu, S.S.; Wei, M.H.; Qiao, T.G. A PC–PU nanoparticle/PU/decellularized scaffold composite vascular patch: Synergistically optimized overall performance promotes endothelialization. Colloids Surf. B Biointerfaces 2017, 160, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Qian, B.; Zhang, W. Protein adsorption resistance of PVP-modified polyurethane film prepared by surface-initiated atom transfer radical polymerization. Appl. Surf. Sci. 2016, 363, 483–489. [Google Scholar] [CrossRef]
- Xu, Q.; Peng, J.; Zhang, W. Electrospun cellulose acetate/P(DMDAAC-AM) nanofibrous membranes for dye adsorption. J. Appl. Polym. Sci. 2020, 137, 48565. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Gao, D. Research on self-degradation of RGO/TiO2-P(AM-DAC) organic-inorganic composite flocculant prepared by surface initiated polymerization and its flocculation mechanism of oil sand tailings. Eur. Polym. J. 2019, 120, 109165. [Google Scholar] [CrossRef]
- Chua, S.C.; Chong, F.K.; Ul Mustafa, M.R.; Mohamed, S.R.; Sujarwo, W.; Abdul, M.M.; Show, P.L.; Ho, Y.C. Microwave radiation-induced grafting of 2-methacryloyloxyethyl trimethyl ammonium chloride onto lentil extract (LE-g-DMC) as an emerging high-performance plant-based grafted coagulant. Sci. Rep. 2020, 10, 3959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Liu, Z.; Zhang, N. Super hydrophilicity of hydroxy modified poly (M-Phenylenediamine) aerogel for separation of oil/water and biocompatibility. Mater. Res. Express. 2018, 5, 045301. [Google Scholar] [CrossRef]
- Hoang, B.N.; Nguyen, T.T.; Bui, Q.P.T. Enhanced selective adsorption of cation organic dyes on polyvinyl alcohol/agar/maltodextrin water-resistance biomembrane. J. Appl. Polym. Sci. 2019, 137, 48904. [Google Scholar] [CrossRef]
- Lien, C.C.; Chen, P.J.; Venault, A. Zwitterionic interpenetrating network for improving the blood compatibility of polypropylene membranes applied to leukodepletion. J. Membr. Sci. 2019, 584, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Chen, Q.; Liu, X.; Yuan, B.; Wu, S.S.; Shen, J.; Lin, S.C. Grafting of zwitterion from cellulose membranes via ATRP for improving blood compatibility. Biomacromolecules 2009, 10, 2809–2816. [Google Scholar] [CrossRef]
- Beilis, E.; Belgorodsky, B.; Fadeev, L.; Cohen, H.; Richter, S. Surface-induced conformational changes in doped bovine serum albumin self-assembled monolayers. J. Am. Chem. Soc. 2014, 136, 6151–6154. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, M.; Miao, R. Effect of monovalent cations on ultrafiltration membrane fouling of protein. China Environ. Sci. 2017, 37, 1792–1797. [Google Scholar]
- Refaai, M.A.; Phipps, R.P.; Spinelli, S.L.; Blumberg, N. Platelet transfusions: Impact on hemostasis, thrombosis, inflammation and clinical outcomes. Thromb. Res. 2011, 127, 287–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Ning, J.P. Synthesis and biocompatibility of an argatroban-modified polysulfone membrane that directly inhibits thrombosis. J. Mater. Sci. Mater. Med. 2018, 29, 66. [Google Scholar] [CrossRef]
- Chi, C.; Sun, B.; Zhou, N.; Zhang, M.; Chu, X.; Yuan, P.; Shen, J. Anticoagulant polyurethane substrates modified with poly (2-methacryloyloxyethyl phosphorylcholine) via SI-RATRP. Colloids Surf. B Biointerfaces 2018, 163, 301–308. [Google Scholar] [CrossRef]
- Habimana, O.; Semiao, A.J.C.; Casey, E. The role of cell-surface interactions in bacterial initial adhesion and consequent biofilm formation on nanofiltration/reverse osmosis membranes. J. Membr. Sci. 2014, 454, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Burton, E.A.; Simon, K.A.; Hou, S.; Ren, D.; Luk, Y.Y. Molecular gradients of bioinertness reveal a mechanistic difference between mammalian cell adhesion and bacterial biofilm formation. Langmuir 2009, 25, 1547–1553. [Google Scholar] [CrossRef]









| Sample ID | Graft Density, μg/cm2 |
|---|---|
| PU-10 | 66 |
| PU-20 | 86 |
| PU-30 | 112 |
| PU-40 | 133 |
| PU-60 | 167 |
| Sample ID | Element Content, at% | ||||
|---|---|---|---|---|---|
| C | O | N | Br | C/O | |
| PU | 70.79 | 27.04 | 1.78 | / | 2.61 |
| PU-NCO | 78.75 | 10.06 | 11.19 | / | 7.82 |
| PU-Br | 76.08 | 14.84 | 8.32 | 0.76 | 5.13 |
| PU-PDMC | 69.35 | 24.91 | 5.74 | / | 2.78 |
| PU-PDAC | 69.76 | 23.26 | 6.83 | 0.05 | 3.00 |
| PU-PDMDAAC | 69.41 | 24.33 | 6.19 | 0.07 | 2.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Zhou, H.; Lu, J.; Huang, S.; Zhu, H.; Li, L. Theoretical and Experimental Optimization of the Graft Density of Functionalized Anti-Biofouling Surfaces by Cationic Brushes. Membranes 2020, 10, 431. https://doi.org/10.3390/membranes10120431
Ren Y, Zhou H, Lu J, Huang S, Zhu H, Li L. Theoretical and Experimental Optimization of the Graft Density of Functionalized Anti-Biofouling Surfaces by Cationic Brushes. Membranes. 2020; 10(12):431. https://doi.org/10.3390/membranes10120431
Chicago/Turabian StyleRen, Yijie, Hongxia Zhou, Jin Lu, Sicheng Huang, Haomiao Zhu, and Li Li. 2020. "Theoretical and Experimental Optimization of the Graft Density of Functionalized Anti-Biofouling Surfaces by Cationic Brushes" Membranes 10, no. 12: 431. https://doi.org/10.3390/membranes10120431