Optical Analysis of the Internal Void Structure in Polymer Membranes for Gas Separation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Fractional Free Volume Measuments
3.2. Voids Complexity
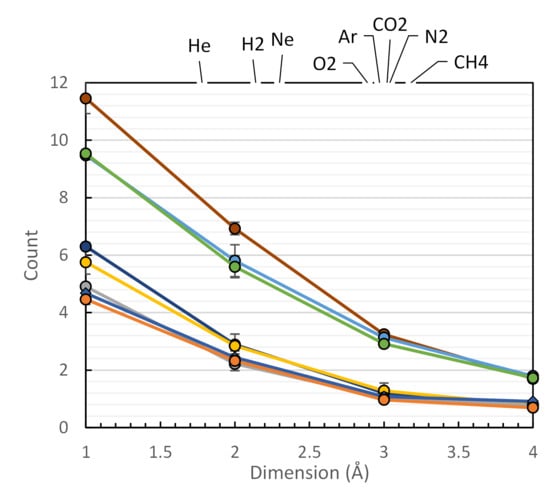
3.3. Voids Shape
3.4. Void Analysis and Selective Separations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yampolskii, Y. Polymeric Gas Separation Membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Gubbins, K.E.; Moore, J.D. Molecular Modeling of Matter: Impact and Prospects in Engineering. Ind. Eng. Chem. Res. 2010, 49, 3026–3046. [Google Scholar] [CrossRef]
- Rizzuto, C.; Caravella, A.; Brunetti, A.; Park, C.H.; Lee, Y.M.; Drioli, E.; Barbieri, G.; Tocci, E. Sorption and Diffusion of CO2/N2 in gas mixture in thermally-rearranged polymeric membranes: A molecular investigation. J. Membr. Sci. 2017, 528, 135–146. [Google Scholar] [CrossRef]
- Kupgan, G.; Abbott, L.J.; Hart, K.E.; Colina, C.M. Modeling Amorphous Microporous Polymers for CO2 Capture and Separations. Chem. Rev. 2018, 118, 5488–5538. [Google Scholar] [CrossRef]
- Vergadou, N.; Theodorou, D.N. Molecular Modeling Investigations of Sorption and Diffusion of Small Molecules in Glassy Polymers. Membranes 2019, 9, 98. [Google Scholar] [CrossRef] [Green Version]
- Neyertz, S.; Douanne, A.; Brown, D. A molecular dynamics simulation study of surface effects on gas permeation in free-standing polyimide membranes. J. Membr. Sci. 2006, 280, 517–529. [Google Scholar] [CrossRef]
- Ebro, H.; Kim, Y.M.; Kim, J.H. Molecular dynamics simulations in membrane-based water treatment processes: A systematic overview. J. Membr. Sci. 2013, 438, 112–125. [Google Scholar] [CrossRef]
- Ridgway, H.F.; Orbell, J.; Gray, S. Molecular simulations of polyamide membrane materials used in desalination and water reuse applications: Recent developments and future prospects. J. Membr. Sci. 2017, 524, 436–448. [Google Scholar] [CrossRef]
- Charpentier, J.-C. Among the trends for a modern chemical engineering, the third paradigm: The time and length multiscale approach as an efficient tool for process intensification and product design and engineering. Chem. Eng. Res. Des. 2010, 88, 248–254. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: New York, NY, USA, 1975. [Google Scholar]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-Tuned Intrinsically Ultramicroporous Polymers Redefine the Permeability/Selectivity Upper Bounds of Membrane-Based Air and Hydrogen Separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef] [Green Version]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.-C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef] [Green Version]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000, 75, 181–196. [Google Scholar] [CrossRef]
- Robeson, L.M.; Smith, Z.P.; Freeman, B.D.; Paul, D.R. Contributions of diffusion and solubility selectivity to the upper bound analysis for glassy gas separation membranes. J. Membr. Sci. 2014, 453, 71–83. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Yampolskii, Y.P. Methods for investigation of the free volume in polymers. Russ. Chem. Rev. 2007, 76, 59–78. [Google Scholar] [CrossRef]
- Greenfield, M.L.; Theodorou, D.N. Geometric analysis of diffusion pathways in glassy and melt atactic polypropylene. Macromolecules 1993, 26, 5461–5472. [Google Scholar] [CrossRef]
- Molecular Dynamics Simulation of Penetrant Diffusion in Amorphous Polypropylene: Diffusion Mechanisms and Simulation Size Effects Macromolecules. Available online: https://pubs.acs.org/doi/pdfplus/10.1021/ma980997e?src=recsys (accessed on 24 October 2020).
- in ‘t Veld, P.J.; Stone, M.T.; Truskett, T.M.; Sanchez, I.C. Liquid Structure via Cavity Size Distributions. J. Phys. Chem. B 2000, 104, 12028–12034. [Google Scholar] [CrossRef]
- Willmore, F.T.; Wang, X.; Sanchez, I.C. Free volume properties of model fluids and polymers: Shape and connectivity. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1385–1393. [Google Scholar] [CrossRef]
- Arizzi, S.; Mott, P.H.; Suter, U.W. Space available to small diffusants in polymeric glasses: Analysis of unoccupied space and its connectivity. J. Polym. Sci. Part B Polym. Phys. 1992, 30, 415–426. [Google Scholar] [CrossRef]
- Dodd, L.R.; Theodorou, D.N. Analytical treatment of the volume and surface area of molecules formed by an arbitrary collection of unequal spheres intersected by planes. Mol. Phys. 1991, 72, 1313–1345. [Google Scholar] [CrossRef]
- Wang, C.; Hirade, T.; Maurer, F.; Eldrup, M.; Pedersen, N. Free-volume distribution and positronium formation in amorphous polymers: Temperature and positron-irradiation-time dependence. J. Chem. Phys. 1998, 108, 4654–4661. [Google Scholar] [CrossRef]
- Hofmann, D.; Heuchel, M.; Yampolskii, Y.; Khotimskii, V.; Shantarovich, V. Free Volume Distributions in Ultrahigh and Lower Free Volume Polymers: Comparison between Molecular Modeling and Positron Lifetime Studies. Macromolecules 2002, 35, 2129–2140. [Google Scholar] [CrossRef]
- Stoyan, D.; Okabe, A.; Boots, B. Sugihara: Spatial Tessellations. Concepts and Applications of Voronoi Diagrams. J. Wiley and Sons, Chichester, New York, Brisbane, Toronto and Singapore 1992, pp. 532, £49.95. Biom. J. 1994, 36, 146. [Google Scholar] [CrossRef]
- Voloshin, V.P.; Medvedev, N.N.; Andrews, M.N.; Burri, R.R.; Winter, R.; Geiger, A. Volumetric Properties of Hydrated Peptides: Voronoi–Delaunay Analysis of Molecular Simulation Runs. J. Phys. Chem. B 2011, 115, 14217–14228. [Google Scholar] [CrossRef]
- Voyiatzis, E.; Böhm, M.C.; Müller-Plathe, F. A geometric approach to identify cavities in particle systems. Comput. Phys. Commun. 2015, 196, 247–254. [Google Scholar] [CrossRef]
- Theodorou, D.N.; Suter, U.W. Shape of unperturbed linear polymers: Polypropylene. Macromolecules 1985, 18, 1206–1214. [Google Scholar] [CrossRef]
- Jiang, Y.; Willmore, F.T.; Sanders, D.; Smith, Z.P.; Ribeiro, C.P.; Doherty, C.M.; Thornton, A.; Hill, A.J.; Freeman, B.D.; Sanchez, I.C. Cavity size, sorption and transport characteristics of thermally rearranged (TR) polymers. Polymer 2011, 52, 2244–2254. [Google Scholar] [CrossRef]
- Park, H.B.; Jung, C.H.; Lee, Y.M.; Hill, A.J.; Pas, S.J.; Mudie, S.T.; Wagner, E.V.; Freeman, B.D.; Cookson, D.J. Polymers with Cavities Tuned for Fast Selective Transport of Small Molecules and Ions. Science 2007, 318, 254–258. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [Green Version]
- Fuoco, A.; Rizzuto, C.; Tocci, E.; Monteleone, M.; Esposito, E.; Budd, P.M.; Carta, M.; Comesaña-Gándara, B.; McKeown, N.B.; Jansen, J.C. The origin of size-selective gas transport through polymers of intrinsic microporosity. J. Mater. Chem. A 2019, 7, 20121–20126. [Google Scholar] [CrossRef]
- Park, C.H.; Tocci, E.; Kim, S.; Kumar, A.; Lee, Y.M.; Drioli, E. A Simulation Study on OH-Containing Polyimide (HPI) and Thermally Rearranged Polybenzoxazoles (TR-PBO): Relationship between Gas Transport Properties and Free Volume Morphology. J. Phys. Chem. B 2014, 118, 2746–2757. [Google Scholar] [CrossRef]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 230. [Google Scholar] [CrossRef]
- Budd, P.; Msayib, K.; Tattershall, C.; Ghanem, B.; Reynolds, K.; Mckeown, N.; Fritsch, D. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- Arcella, V.; Colaianna, P.; Maccone, P.; Sanguineti, A.; Gordano, A.; Clarizia, G.; Drioli, E. A study on a perfluoropolymer purification and its application to membrane formation. J. Membr. Sci. 1999, 163, 203–209. [Google Scholar] [CrossRef]
- Prabhakar, R.S.; Freeman, B.D.; Roman, I. Gas and Vapor Sorption and Permeation in Poly(2,2,4-trifluoro-5-trifluoromethoxy-1,3-dioxole-co-tetrafluoroethylene). Macromolecules 2004, 37, 7688–7697. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Xu, J. Gas Transport Properties of Novel Cardo Poly(aryl ether ketone)s with Pendant Alkyl Groups. Macromolecules 2000, 33, 5672–5679. [Google Scholar] [CrossRef]
- Jansen, J.C.; Drioli, E. Poly (ether ether ketone) derivative membranes—A review of their preparation, properties and potential. Polym. Sci. Ser. A 2009, 51, 1355. [Google Scholar] [CrossRef]
- Esposito, E.; Bruno, R.; Monteleone, M.; Fuoco, A.; Ferrando Soria, J.; Pardo, E.; Armentano, D.; Jansen, J.C. Glassy PEEK-WC vs. Rubbery Pebax® 1657 Polymers: Effect on the Gas Transport in CuNi-MOF Based Mixed Matrix Membranes. Appl. Sci. 2020, 10, 1310. [Google Scholar] [CrossRef] [Green Version]
- Fuoco, A.; Satilmis, B.; Uyar, T.; Monteleone, M.; Esposito, E.; Muzzi, C.; Tocci, E.; Longo, M.; De Santo, M.P.; Lanč, M.; et al. Comparison of pure and mixed gas permeation of the highly fluorinated polymer of intrinsic microporosity PIM-2 under dry and humid conditions: Experiment and modelling. J. Membr. Sci. 2020, 594, 117460. [Google Scholar] [CrossRef]
- Satilmis, B.; Lanč, M.; Fuoco, A.; Rizzuto, C.; Tocci, E.; Bernardo, P.; Clarizia, G.; Esposito, E.; Monteleone, M.; Dendisová, M.; et al. Temperature and pressure dependence of gas permeation in amine-modified PIM-1. J. Membr. Sci. 2018, 555, 483–496. [Google Scholar] [CrossRef]
- Tocci, E.; Hofmann, D.; Paul, D.; Russo, N.; Drioli, E. A molecular simulation study on gas diffusion in a dense poly(ether–ether–ketone) membrane. Polymer 2001, 42, 521–533. [Google Scholar] [CrossRef]
- Tocci, E.; Pullumbi, P. Molecular simulation of realistic membrane models of alkylated PEEK membranes. Mol. Simul. 2006, 32, 145–154. [Google Scholar] [CrossRef]
- Macchione, M.; Jansen, J.C.; Tocci, E.; Drioli, E. Influence of residual solvent on the gas transport properties of dense Hyflon® AD 60X gas separation membranes. Desalination 2006, 200, 49–51. [Google Scholar] [CrossRef]
- Jansen, J.C.; Macchione, M.; Tocci, E.; De Lorenzo, L.; Yampolskii, Y.P.; Sanfirova, O.; Shantarovich, V.P.; Heuchel, M.; Hofmann, D.; Drioli, E. Comparative Study of Different Probing Techniques for the Analysis of the Free Volume Distribution in Amorphous Glassy Perfluoropolymers. Macromolecules 2009, 42, 7589–7604. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA; Materials Studio 7.0.; Dassault Systèmes: San Diego, CA, USA, 2013.
- Eaton, J.W.; Bateman, D.; Hauberg, S.; Wehbring, R. GNU Octave Manual: A High-Level Interactive Language for Numerical Computations; A High-Level Interactive Language for Numerical Computations. Edition 5 for Octave Version 5.2.0. 2019. Available online: https://octave.org/octave.pdf (accessed on 24 October 2020).
- Various Authors. Package Image, Image Processing. 2020. Available online: https://octave.sourceforge.io/image/function/regionprops.html (accessed on 24 October 2020).
- Hofmann, D.; Entrialgo-Castano, M.; Lerbret, A.; Heuchel, M.; Yampolskii, Y. Molecular Modeling Investigation of Free Volume Distributions in Stiff Chain Polymers with Conventional and Ultrahigh Free Volume: Comparison between Molecular Modeling and Positron Lifetime Studies. Macromolecules 2003, 36, 8528–8538. [Google Scholar] [CrossRef]
- Materials Studio Guide; Atom volumes & surfaces; Accelrys Software Inc.: San Diego, CA, USA, 2008.
- Connolly, M.L. Analytical molecular surface calculation. J. Appl. Crystallogr. 1983, 16, 548–558. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Du, N.; Robertson, G.P.; Song, J.; Pinnau, I.; Thomas, S.; Guiver, M.D. Polymers of Intrinsic Microporosity Containing Trifluoromethyl and Phenylsulfone Groups as Materials for Membrane Gas Separation†. Macromolecules 2008, 41, 9656–9662. [Google Scholar] [CrossRef] [Green Version]
- Freeman, B.; Yampolskii, Y.; Pinnau, I. Materials Science of Membranes for Gas and Vapor Separation; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 978-0-470-02904-6. [Google Scholar]
- Ismail, A.F.; Khulbe, K.; Matsuura, T. Gas Separation Membranes: Polymeric and Inorganic; Springer International Publishing: Berlin, Germany, 2015; ISBN 978-3-319-01094-6. [Google Scholar]
- Teplyakov, V.; Meares, P. Correlation aspects of the selective gas permeabilities of polymeric materials and membranes. Gas Sep. Purif. 1990, 4, 66–74. [Google Scholar] [CrossRef]







| Name | Structure | Simulation Boxes Lateral Dimension (nm) | References |
|---|---|---|---|
| PIM-1 |  | 3.999 ± 0.004 | [39] |
| PIM-2 |  | 4.33 ± 0.03 | [45] |
| PIM-NH2 |  | 3.9105 ± 0.0045 | [46] |
| PEEK-WC |  | 3.90104 ± 0.00002 | [47,48] |
| DMPEEK |  | 3.97634 ± 0.00001 | [48] |
| TMPEEK |  | 4.103847 ± 0.000005 | [48] |
| Hyflon® AD60x |  with x = 0.6 with x = 0.6 | 4.92 ± 0.04 | [49,50] |
| Hyflon® AD80x |  with x = 0.8 with x = 0.8 | 4.953 ± 0.004 | [49,50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzzi, C.; Fuoco, A.; Monteleone, M.; Esposito, E.; Jansen, J.C.; Tocci, E. Optical Analysis of the Internal Void Structure in Polymer Membranes for Gas Separation. Membranes 2020, 10, 328. https://doi.org/10.3390/membranes10110328
Muzzi C, Fuoco A, Monteleone M, Esposito E, Jansen JC, Tocci E. Optical Analysis of the Internal Void Structure in Polymer Membranes for Gas Separation. Membranes. 2020; 10(11):328. https://doi.org/10.3390/membranes10110328
Chicago/Turabian StyleMuzzi, Chiara, Alessio Fuoco, Marcello Monteleone, Elisa Esposito, Johannes C. Jansen, and Elena Tocci. 2020. "Optical Analysis of the Internal Void Structure in Polymer Membranes for Gas Separation" Membranes 10, no. 11: 328. https://doi.org/10.3390/membranes10110328