Hyperbranch-Crosslinked S-SEBS Block Copolymer Membranes for Desalination by Pervaporation
Abstract
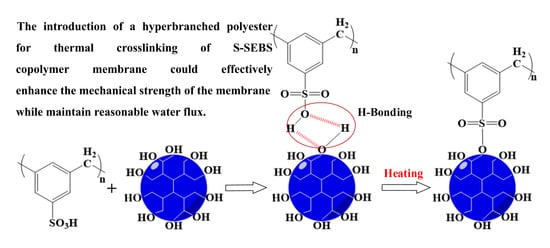
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Pervaporation Test
3. Results and Discussion
3.1. Characterization of H302/S-SEBS Membrane
3.1.1. Crosslinking Reaction
3.1.2. Microstructure of Membranes
3.1.3. Hydrophilicity: Water Contact Angle, IEC, Water Uptake and Swelling Ratio
3.1.4. Mechanical and Thermal Properties
3.2. Pervaporation Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Bolto, B.; Hoang, M.; Xie, Z. Desalination by pervaporation: A review. Desalination 2016, 387, 46–60. [Google Scholar] [CrossRef]
- Xie, Z.; Hoang, M.; Duong, T.; Ng, D.; Dao, B.; Gray, S.R. Sol-gel derived poly(vinyl alcohol)/maleic acid/silica hybrid membrane for desalination by pervaporation. J. Membr. Sci. 2011, 383, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Huth, E.; Muthu, S.; Ruff, L.; Brant, J.A. Feasibility assessment of pervaporation for desalinating high-salinity brines. J. Water Reuse Desal. 2014, 4, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Hoang, M.; Ng, D.; Doherty, C.; Hill, A.; Gray, S. Effect of heat treatment on pervaporation separation of aqueous salt solution using hybrid PVA/MA/TEOS membrane. Sep. Purif. Technol. 2014, 127, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Ju, H.; Sagle, A.C.; Freeman, B.D.; Mardel, J.I.; Hill, A.J. Characterization of sodium chloride and water transport in crosslinked poly(ethylene oxide) hydrogels. J. Membr. Sci. 2010, 358, 131–141. [Google Scholar] [CrossRef]
- Semenova, S.I.; Ohya, H.; Soontarapa, K. Hydrophilic membranes for pervaporation: An analytical review. Desalination 1997, 110, 251–286. [Google Scholar] [CrossRef]
- Cabasso, I.; Korngold, E.; Liu, Z.Z. On the separation of alcohol water mixtures by polyethylene ion-exchange membranes. J. Polm. Sci. Part. C Polymer Lett. 1985, 23, 557–581. [Google Scholar] [CrossRef]
- Park, H.B.; Freeman, B.D.; Zhang, Z.-B.; Sankir, M.; McGrath, J. Highly chlorine-tolerant polymers for desalination. Angew. Chem. Int. Ed. 2008, 47, 6019–6024. [Google Scholar] [CrossRef]
- Weiss, R.; Sen, A.; Willis, C.; Pottick, L. Block copolymer ionomers: 1. Synthesis and physical properties of sulphonated poly(styrene-ethylene/butylene-styrene). Polymer 1991, 32, 1867–1874. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Kim, S.J.; Oh, D.Y.; Hong, Y.T.; Nam, S. Proton conduction and methanol transport through sulfonated poly (styrene-b-ethylene/butylene -b-styrene)/clay nanocomposite. Macromol. Res. 2011, 19, 84–89. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Y.; Li, N. Preparation, characterization and performance of sulfonated poly (styrene-ethylene/butylene-styrene) block copolymer membranes for water desalination by pervaporation. Desalination 2016, 390, 33–46. [Google Scholar] [CrossRef]
- Hou, H.; Di Vona, M.L.; Knauth, P. Building bridges: Crosslinking of sulfonated aromatic polymers-A review. J. Membr. Sci. 2012, 423, 113–127. [Google Scholar] [CrossRef]
- Mikhailenko, S.D.; Wang, K.; Kaliaguine, S.; Xing, P.; Robertson, G.P.; Guiver, M.D. Proton conducting membranes based on cross-linked sulfonated poly(ether ether ketone) (SPEEK). J. Membr. Sci. 2004, 233, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Nolte, R.; Ledjeff, K.; Bauer, M.; Mülhaupt, R. Partially sulfonated poly (arylene ether sulfone)—A versatile proton conducting membrane material for modern energy conversion technologies. J. Membr. Sci. 1993, 83, 211–220. [Google Scholar] [CrossRef]
- Kaur, S.; Florio, G.; Michalak, D. Cross-linking of sulfonated styrene–ethylenebutylene–styrene triblock polymer via sulfonamide linkages. Polymer 2002, 43, 5163–5167. [Google Scholar] [CrossRef]
- Park, H.B.; Lee, C.H.; Sohn, J.Y.; Lee, Y.M.; Freeman, B.D.; Kim, H.J. Effect of crosslinked chain length in sulfonated polyimide membranes on water sorption, proton conduction, and methanol permeation properties. J. Membr. Sci. 2006, 285, 432–443. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Wei, X.-Z.; Liu, X.-F.; Zhu, L.-P.; Zhu, B.-K.; Wei, Y.-F.; Xu, Y.-Y. Preparation and pervaporation properties of crosslinked hyperbranched poly(amine-ester) membranes. J. Membr. Sci. 2008, 307, 292–298. [Google Scholar] [CrossRef]
- Luo, Y.; Xin, W.; Li, G.; Yang, Y.; Liu, J.; Lv, Y.; Jiu, Y. Pervaporation properties of EC membrane crosslinked by hyperbranched-polyester acrylate. J. Membr. Sci. 2007, 303, 183–193. [Google Scholar] [CrossRef]
- Sun, D.; Yang, P.; Sun, H.-L.; Li, B. Preparation and characterization of cross-linked poly (vinyl alcohol)/hyperbranched polyester membrane for the pervaporation dehydration of ethylene glycol solution. Eur. Polym. J. 2015, 62, 155–166. [Google Scholar] [CrossRef]
- Shi, Y.; Eze, C.; Xiong, B.; He, W.; Zhang, H.; Lim, T.; Ukil, A.; Zhao, J. Recent development of membrane for vanadium redox flow battery applications: A review. Appl. Energy 2019, 238, 202–224. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Koh, H.C.; Rhim, J.W.; Nam, S.Y. Preparation of sulfonated SEBS block copolymer membranes and their permeation properties. Desalination 2008, 233, 173–182. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.; Jung, B. Morphology and transport properties of protons and methanol through partially sulfonated block copolymers. J. Memb. Sci. 2005, 250, 175–182. [Google Scholar] [CrossRef]
- Storey, R.F.; Iii, D.W.B. Poly(styrene-b-isobutylene-b-styrene) block copolymers and ionomers therefrom: Morphology as determined by small-angle X-ray scattering and transmission electron microscopy. Polymer 2000, 41, 3205–3211. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.; Jung, B. Proton conductivities and methanol permeabilities of membranes made from partially sulfonated polystyrene-block-polystyrene copolymers. J. Membr. Sci. 2002, 207, 129–137. [Google Scholar] [CrossRef]
- Choi, J.-H.; Willis, C.L.; Winey, K.I. Structure-property relationship in sulfonated pentablock copolymers. J. Membr. Sci. 2012, 394, 169–174. [Google Scholar] [CrossRef]
- Kang, E.-T.; Tan, K.L.; Kato, K.; Uyama, Y.; Ikada, Y. Surface modification and functionalization of polytetrafluoroethylene films. Macromolecules 1996, 29, 6872–6879. [Google Scholar] [CrossRef]
- Doganci, M.D.; Cansoy, C.E.; Ucar, I.O.; Erbil, H.Y.; Mielczarski, E.; Mielczarski, J. Combined XPS and contact angle studies of flat and rough ethylene-vinyl acetate copolymer films. J. Appl. Polym. Sci. 2012, 124, 2100–2109. [Google Scholar] [CrossRef]
- Yen, S.P.S.; Narayanan, S.R.; Halpert, G.; Graham, E.; Yavrouian, A. Polymer Material for Electrolytic Membranes in Fuel Cells. U.S. Patent 5,795,496, 18 August 1998. [Google Scholar]
- Dai, J.; He, G.; Ruan, X.; Zheng, W.; Pan, Y.; Yan, X. Constructing a rigid crosslinked structure for enhanced conductivity of imidazolium functionalized polysulfone hydroxide exchange membrane. Int. J. Hydrogen Energy 2016, 41, 10923–10934. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.; Lu, Y.; Li, N.; Zeng, F.; Wang, Q.; Bai, H.; Xie, Z. Hyperbranch-Crosslinked S-SEBS Block Copolymer Membranes for Desalination by Pervaporation. Membranes 2020, 10, 277. https://doi.org/10.3390/membranes10100277
Yan M, Lu Y, Li N, Zeng F, Wang Q, Bai H, Xie Z. Hyperbranch-Crosslinked S-SEBS Block Copolymer Membranes for Desalination by Pervaporation. Membranes. 2020; 10(10):277. https://doi.org/10.3390/membranes10100277
Chicago/Turabian StyleYan, Mengyu, Yunyun Lu, Na Li, Feixiang Zeng, Qinzhuo Wang, Hongcun Bai, and Zongli Xie. 2020. "Hyperbranch-Crosslinked S-SEBS Block Copolymer Membranes for Desalination by Pervaporation" Membranes 10, no. 10: 277. https://doi.org/10.3390/membranes10100277