Performance Evaluation and Kinetic Analysis of Photocatalytic Membrane Reactor in Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
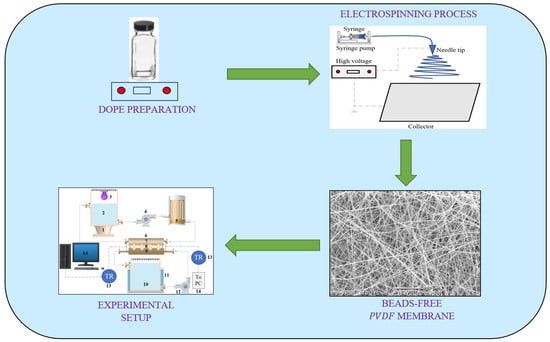
2.1. Membrane Fabrication
2.2. Membrane Characterization and Analysis
2.2.1. Membrane Morphology
2.2.2. Surface Zeta Potential
2.2.3. Membrane Contact Angle
2.3. TiO2 Characterization and Analysis
2.4. Experimental Setup
2.5. Experimental Procedures
2.6. Data Representation and Analysis
Membrane Flux and Dye Removal Efficiency
3. Results and Discussion
3.1. Membrane Distillation
3.2. PMR Performance
3.3. Feed Concentration Analysis
3.4. Photocatalysis Kinetic Analysis
3.5. Comparison between PMRs and Previous Studies
4. Remarks and Conclusions
- Electrospinning conditions adopted in the current study enable the acquisition of free beads fibers with a negatively charged surface and a high hydrophobicity membrane.
- The performance of the PMR exceeds the conventional MD, thus allowing more water to be reused which is an important advantage from the economic and environmental points of view.
- Using the MD is preferable with high MB concentrations (i.e., and ) to obtain nearly pure permeate besides recovering dyes from the concentrate.
- Almost separation efficiency was achieved by operating the PMR at the investigated optimum conditions, which provides high-quality water and low-dyeing waste concentration suitable for discharge.
- Photodegradation of MB on behaves similarly to the pseudo-first-order kinetic model.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols | |
| Membrane area () | |
| Initial feed concentration () | |
| Permeate concentration ( | |
| Permeate flux () | |
| Pseudo-first-order rate constant () | |
| Pseudo-second-order rate constant | |
| Molecular weight () | |
| Permeate mass () | |
| Calculated adsorption capacity | |
| Equilibrium adsorption capacity | |
| Adsorption capacity at time | |
| Correlation coefficient | |
| Sampling time () | |
| Greek letters | |
| Dye removal efficiency () | |
| Abbreviations | |
| Silver | |
| Air Gap Membrane distillation | |
| Aminopropyltriethoxysilane | |
| Cadmium Sulfide | |
| Cerium Dioxide | |
| Direct Contact Membrane Distillation | |
| N-Dimethyl Acetamide | |
| Dimethyl Acetamide-Sodium Dodecyl Sulphate-Graphene Oxide | |
| Ferric Oxide | |
| Fourier Transform Infrared Spectrometer | |
| Potassium Chloride | |
| Potassium Hydroxide | |
| MB | Methylene Blue |
| MD | Membrane Distillation |
| Multi-Walled Carbon Nanotubes | |
| Non-solvent Induced Phase Separation | |
| Poly-methyl Methacrylate | |
| Photocatalytic Membrane Reactors | |
| Poly-Vinylidene Flouride | |
| Poly-Vinyl Propylene | |
| Scanning Electron Microscope | |
| Sweep Gas Membrane Distillation | |
| Titanium Dioxide | |
| Trifluoro Ethylene | |
| Ultraviolet | |
| Vacuum Membrane Distillation | |
| Vanadium Oxide | |
| Tungsten Trioxide | |
| X-Ray Diffraction | |
| Zinc Oxide | |
| ZnS | Zinc Sulfide |
| Zirconium Dioxide | |
References
- Desa, A.L.; Hairom, N.H.H.; Ng, L.Y.; Ng, C.Y.; Ahmad, M.K.; Mohammad, A.W. Industrial textile wastewater treatment via membrane photocatalytic reactor (MPR) in the presence of ZnO-PEG nanoparticles and tight ultrafiltration. J. Water Process. Eng. 2019, 31, 100872. [Google Scholar] [CrossRef]
- Mondal, S.; Purkait, M.K.; De, S. Advances in Dye Removal Technologies; Springer: Singapore, 2018. [Google Scholar]
- Molinari, R.; Pirillo, F.; Falco, M.; Loddo, V.; Palmisano, L. Photocatalytic degradation of dyes by using a membrane reactor. Chem. Eng. Process. Process Intensif. 2004, 43, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Basile, A.; Gallucci, F. Membranes for Membrane Reactors: Preparation, Optimization and Selection; John Wiley & Sons: West Sussex, UK, 2010. [Google Scholar]
- Elmarghany, M.R.; El-Shazly, A.H.; Rajabzadeh, S.; Salem, M.S.; Shouman, M.A.; Sabry, M.N.; Matsuyama, H.; Nady, N. Triple-layer nanocomposite membrane prepared by electrospinning based on modified pes with carbon nanotubes for membrane distillation applications. Membranes 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashoor, B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review. Desalination 2016, 398, 222–246. [Google Scholar] [CrossRef]
- El-Marghany, M.R.; El-Shazly, A.H.; Salem, M.S.A.; Sabry, M.N.; Nady, N. Novel membrane suitable for membrane distillation: Effect of mixed nanofillers on the membrane performance. Key Eng. Mater. 2019, 801, 325–330. [Google Scholar] [CrossRef]
- Ali, I.; Bamaga, O.; Gzara, L.; Bassyouni, M.; Abdel-Aziz, M.; Soliman, M.; Drioli, E.; Albeirutty, M. Assessment of blend PVDF membranes, and the effect of polymer concentration and blend composition. Membranes 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Mat Nawi, N.I.; Chean, H.M.; Shamsuddin, N.; Bilad, M.R.; Narkkun, T.; Faungnawakij, K.; Khan, A.L. Development of hydrophilic PVDF membrane using vapour induced phase separation method for produced water treatment. Membranes 2020, 10, 121. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, X.; Chen, S.; Zhao, H.; Zhao, Y. Fabrication of photocatalytic membrane and evaluation its efficiency in removal of organic pollutants from water. Sep. Purif. Technol. 2006, 50, 147–155. [Google Scholar] [CrossRef]
- Van Gerven, T.; Mul, G.; Moulijn, J.; Stankiewicz, A. A review of intensification of photocatalytic processes. Chem. Eng. Process. Process Intensif. 2007, 46, 781–789. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: State of the art and present applications in honor of Pr. RL Burwell Jr. (1912–2003), Former Head of Ipatieff Laboratories, Northwestern University, Evanston (Ill). Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Buscio, V.; Brosillon, S.; Mendret, J.; Crespi, M.; Gutiérrez-Bouzán, C. Photocatalytic membrane reactor for the removal of CI Disperse red 73. Materials 2015, 8, 3633–3647. [Google Scholar] [CrossRef] [Green Version]
- Ollis, D.F. Integrating photocatalysis and membrane technologies for water treatment. Ann. N. Y. Acad. Sci. 2003, 984, 65–84. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P.; Szymański, K.; Darowna, D.; Mozia, S. Overview of photocatalytic membrane reactors in organic synthesis, energy storage and environmental applications. Catalysts 2019, 9, 239. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.S.A.; El-Shazly, A.H.; El-Marghany, M.R.; Sabry, M.N.; Nady, N. Effect of Adding Functionalized Graphene on the Performance of PVDF Membrane in Direct Contact Membrane Distillation. Key Eng. Mater. 2019, 801, 337–342. [Google Scholar] [CrossRef]
- Grzechulska, J.; Morawski, A.W. Photocatalytic decomposition of azo-dye acid black 1 in water over modified titanium dioxide. Appl. Catal. B 2002, 36, 45–51. [Google Scholar] [CrossRef]
- Suriani, A.; Mohamed, A.; Othman, M.; Rohani, R.; Yusoff, I.; Mamat, M.; Hashim, N.; Azlan, M.; Ahmad, M.; Marwoto, P. Incorporation of electrochemically exfoliated graphene oxide and TiO2 into polyvinylidene fluoride-based nanofiltration membrane for Dye Rejection. Water Air Soil Pollut. 2019, 230, 176. [Google Scholar] [CrossRef]
- Benhabiles, O.; Galiano, F.; Marino, T.; Mahmoudi, H.; Lounici, H.; Figoli, A. Preparation and characterization of TiO2-PVDF/PMMA blend membranes using an alternative non-toxic solvent for UF/MF and photocatalytic application. Molecules 2019, 24, 724. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.M.; Ribeiro, J.M.; Teixeira, S.; Petrovykh, D.; Cuniberti, G.; Pereira, L.; Lanceros-Méndez, S. Photocatalytic microporous membrane against the increasing problem of water emerging pollutants. Materials 2019, 12, 1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-G.; Javed, H.; Zhang, D.; Kim, J.-H.; Westerhoff, P.; Li, Q.; Alvarez, P.J. Porous electrospun fibers embedding TiO2 for adsorption and photocatalytic degradation of water pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Ayodele, B.V.; Mansor, W.N.W.; Abdullah, S. Effect of incorporating TiO2 photocatalyst in pvdf hollow fibre membrane for photo-assisted degradation of methylene blue. Bull. Chem. React. Eng. Catal. 2018, 13, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Pu, H. A facile method to prepare polyvinylidene fluoride composite nanofibers with high photocatalytic activity via nanolayer coextrusion. Eur. Polym. J. 2018, 99, 361–367. [Google Scholar] [CrossRef]
- Galiano, F.; Song, X.; Marino, T.; Boerrigter, M.; Saoncella, O.; Simone, S.; Faccini, M.; Chaumette, C.; Drioli, E.; Figoli, A. Novel photocatalytic PVDF/Nano-TiO2 hollow fibers for environmental remediation. Polymers 2018, 10, 1134. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Tian, Y.; Zhang, J.; Sun, Z.; Zhao, J.; Zhang, J.; Zuo, W. Precisely-controlled modification of PVDF membranes with 3D TiO2/ZnO nanolayer: Enhanced anti-fouling performance by changing hydrophilicity and photocatalysis under visible light irradiation. J. Membr. Sci. 2017, 528, 359–368. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, Z.; Pan, Y.; Zeng, G. Antibacterial photocatalytic self-cleaning poly (vinylidene fluoride) membrane for dye wastewater treatment. Polym. Adv. Technol. 2018, 29, 254–262. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Seid, M.G.; Choe, J.W.; Kim, E.-J.; Chung, Y.C.; Cho, K.; Lee, C.; Hong, S.W. Highly reusable TiO2 nanoparticle photocatalyst by direct immobilization on steel mesh via PVDF coating, electrospraying, and thermal fixation. Chem. Eng. J. 2016, 306, 344–351. [Google Scholar] [CrossRef]
- Fischer, K.; Grimm, M.; Meyers, J.; Dietrich, C.; Gläser, R.; Schulze, A. Photoactive microfiltration membranes via directed synthesis of TiO2 nanoparticles on the polymer surface for removal of drugs from water. J. Membr. Sci. 2015, 478, 49–57. [Google Scholar] [CrossRef]
- Li, J.-H.; Yan, B.-F.; Shao, X.-S.; Wang, S.-S.; Tian, H.-Y.; Zhang, Q.-Q. Influence of Ag/TiO2 nanoparticle on the surface hydrophilicity and visible-light response activity of polyvinylidene fluoride membrane. Appl. Surf. Sci. 2015, 324, 82–89. [Google Scholar] [CrossRef]
- Jia, L.M.; Wen, C.; Xu, J.Y.; Xiao, C.F. Enhancement of retention and antifouling capability for PVDF UF membrane modified by nano-TiO2 sol. In Proceedings of the Second International Conference on Smart Materials and Nanotechnology in Engineering, Weihai, China, 20 October 2009; p. 74935R. [Google Scholar]
- Soo, J.Z.; Ang, B.C.; Ong, B.H. Microscopic characterization and analysis of electrospun TiO2-PVP and TiO2-PVDF fibers. Solid State Phenom. 2017, 264, 33–37. [Google Scholar] [CrossRef]
- Tan, Y.; Sun, Z.; Meng, H.; Han, Y.; Wu, J.; Xu, J.; Xu, Y.; Zhang, X. A new MOFs/polymer hybrid membrane: MIL-68 (Al)/PVDF, fabrication and application in high-efficient removal of p-nitrophenol and methylene blue. Sep. Purif. Technol. 2019, 215, 217–226. [Google Scholar] [CrossRef]
- Zeitoun, Z.; El-Shazly, A.H.; Nosier, S.R.; Elmarghany, M.; Salem, M.S.; Taha, M.M. Electrospinning of polyvinylidene fluoride membranes: Effect of membrane composition and fabrication conditions. Alex. Eng. J. Under review.
- An, A.K.; Guo, J.; Jeong, S.; Lee, E.-J.; Tabatabai, S.A.A.; Leiknes, T. High flux and antifouling properties of negatively charged membrane for dyeing wastewater treatment by membrane distillation. Water Res. 2016, 103, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latif, S.M.F.A. Photocatalytic Degradation of Methylene Blue Dye Using a Slurry of TiO2 in a Falling Film Reactor. Master’s Thesis, Alexandria University, Alexandria Governorate, Egypt, 2017. [Google Scholar]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase characterization of TiO2 powder by XRD and TEM. Kasetsart J. (Nat. Sci.) 2008, 42, 357–361. [Google Scholar]
- Mozia, S.; Morawski, A.W.; Toyoda, M.; Inagaki, M. Effectiveness of photodecomposition of an azo dye on a novel anatase-phase TiO2 and two commercial photocatalysts in a photocatalytic membrane reactor (PMR). Sep. Purif. Technol. 2008, 63, 386–391. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Liu, W.; Weng, X.; Dong, X.; Zhang, X.; Zhou, W. High Flux and hydrophilic fibrous ultrafiltration membranes based on electrospun titanium dioxide nanoparticles/polyethylene oxide/poly (vinylidene fluoride) composite scaffolds. J. Nanosci. Nanotechnol. 2017, 17, 9042–9049. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Zhu, L. Preparation of novel poly (vinylidene fluoride)/TiO2 photocatalysis membranes for use in direct contact membrane distillation. J. Nanopart. Res. 2018, 20, 63. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Mozia, S.; Toyoda, M.; Inagaki, M.; Tryba, B.; Morawski, A.W. Application of carbon-coated TiO2 for decomposition of methylene blue in a photocatalytic membrane reactor. J. Hazard. Mater. 2007, 140, 369–375. [Google Scholar] [CrossRef]
- Mozia, S.; Toyoda, M.; Tsumura, T.; Inagaki, M.; Morawski, A.W. Comparison of effectiveness of methylene blue decomposition using pristine and carbon-coated TiO2 in a photocatalytic membrane reactor. Desalination 2007, 212, 141–151. [Google Scholar] [CrossRef]
- Boonying, A.; Kasempremsak, A.; Junpirom, S.; Nuchitprasittichai, A. Wetted wall photocatalytic reactor for methylene blue degradation. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012060. [Google Scholar] [CrossRef]
- Rahimi, S.; Poormohammadi, A.; Salmani, B.; Ahmadian, M.; Rezaei, M. Comparing the photocatalytic process efficiency using batch and tubular reactors in removal of methylene blue dye and COD from simulated textile wastewater. J. Water Reuse. Desal. 2016, 6, 574–582. [Google Scholar] [CrossRef] [Green Version]

















| Membrane | Additives | Pollutant | Ref. | |||
|---|---|---|---|---|---|---|
| Type | Fabrication Method | Type | Conc. | Type | Conc. | |
| PVDF | Phase immersion | (: ) 79 wt%:1 wt% | MB | [21] | ||
| PVDF/PMMA | Phase inversion | MB | 10 µmol/L | [22] | ||
| PVDF-TrFE | Solvent casting | MB | [23] | |||
| PVDF-PVP | Electrospinning | MB | [24] | |||
| PVDF | Electrospinning | MB | [25] | |||
| PVDF | Coextrusion | MB | [26] | |||
| PVDF | Coextrusion | TiO2/MWCNTs | TiO2/MWCNTs 10 wt% TiO2/MWCNTs 20 wt% TiO2/MWCNTs 30 wt% TiO2/MWCNTs 40 wt% | MB | [26] | |
| PVDF | Phase inversion | MB | [27] | |||
| PVDF | Purchased | (TiO2:ZnO) 1:1 (TiO2:ZnO) 1:3 (TiO2:ZnO) 1:5 | MB | [28] | ||
| PVDF | Phase inversion | 0.1 g 0.2 g 0.5 g | MB | [29] | ||
| PVDF | Dip coating | MB | [30] | |||
| PVDF | Dip coating | Titanium isopropoxide | MB | [31] | ||
| PVDF | Nonsolvent induced phase separation (NIPS)-immersion precipitation inversion | () : () : () : () : | MB | 10 mg/L | [32] | |
| PVDF | Phase inversion | 0 vol% 6 vol% 12 vol% 21 vol% | MB | 0.01 mmo/L | [33] | |
| Electrospinning Voltage | |
| Flow rate | |
| Spinneret speed | |
| Cleaning frequency | |
| Cleaning interval | |
| Spinning distance |
| MB Concentration (ppm) | Percentage of Dye Removal | |||
|---|---|---|---|---|
| (MD) | ||||
| After 1 h | ||||
| After 2 h | ||||
| After 3 h | ||||
| After 4 h | ||||
| Optimum Conditions | Rate Law | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||||||
| Operating Conditions | Type of Photocatalytic Reactor | ||
|---|---|---|---|
| PMR | WWPR | TR | |
| Photocatalyst | |||
| Photocatalyst loading () | |||
| Initial MB concentration () | |||
| Operating time () | |||
| Removal | 100 | 89.5 | 74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeitoun, Z.; El-Shazly, A.H.; Nosier, S.; Elmarghany, M.R.; Salem, M.S.; Taha, M.M. Performance Evaluation and Kinetic Analysis of Photocatalytic Membrane Reactor in Wastewater Treatment. Membranes 2020, 10, 276. https://doi.org/10.3390/membranes10100276
Zeitoun Z, El-Shazly AH, Nosier S, Elmarghany MR, Salem MS, Taha MM. Performance Evaluation and Kinetic Analysis of Photocatalytic Membrane Reactor in Wastewater Treatment. Membranes. 2020; 10(10):276. https://doi.org/10.3390/membranes10100276
Chicago/Turabian StyleZeitoun, Zeyad, Ahmed H. El-Shazly, Shaaban Nosier, Mohamed R. Elmarghany, Mohamed S. Salem, and Mahmoud M. Taha. 2020. "Performance Evaluation and Kinetic Analysis of Photocatalytic Membrane Reactor in Wastewater Treatment" Membranes 10, no. 10: 276. https://doi.org/10.3390/membranes10100276