Membranes for Modelling Cardiac Tissue Stiffness In Vitro Based on Poly(trimethylene carbonate) and Poly(ethylene glycol) Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis and Characterization of Dimethacrylate-Functionalized PTMC, PEG and PTMC-PEG-PTMC Macromers
2.3. Polymeric Network Formation—Membrane Fabrication
2.4. Characterization of Photo-Crosslinked Networks
2.5. Verapamil Adsorption to the Membranes and PDMS
2.6. Cell Culture Plate Assembly and L-DOPA/Matrigel Double Coating
2.7. Cardiomyocyte Culture on Polymeric Membranes
2.8. Contractility Analysis of the Cardiomyocytes
2.9. Statistical Analysis
3. Results and Discussion
3.1. Macromer Synthesis and Properties
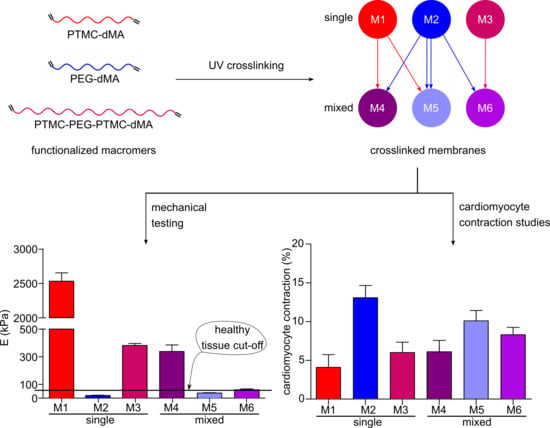
3.2. The Characteristics of the Photo-Crosslinked Membranes Can Be Tailored by Mixing the Macromers
3.3. Verapamil Adsorption to the Membranes
3.4. Cardiomyocyte Contraction Behavior Is Influenced by the Stiffness of the Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, E139–E596. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Carag-Krieger, C.; Johnson, C.P.; Raab, M.; Tang, H.-Y.; Speicher, D.W.; Sanger, J.W.; Sanger, J.M.; Discher, D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating. J. Cell Sci. 2008, 121, 3794–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, R.R.; Herron, T.; Simmons, R.; Shore, D.; Kumar, P.; Sethia, B.; Chua, F.; Vassiliadis, E.; Kentish, J.C. Passive stiffness of myocardium from congenital heart disease and implications for diastole. Circulation 2010, 121, 979–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.C.; Slaats, R.H.; Schwach, V.; Rivera-Arbelaez, J.M.; Tertoolen, L.G.J.; van Meer, B.J.; Molenaar, R.; Mummery, C.L.; Claessens, M.M.A.E.; Passier, R. A cardiomyocyte show of force: A fluorescent alpha-actinin reporter line sheds light on human cardiomyocyte contractility versus substrate stiffness. J. Mol. Cell. Cardiol. 2020, 141, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Volinsky, A.A.; Gallant, N.D. Crosslinking effect on polydimethylsiloxane elastic modulus measured by custom-built compression instrument. J. Appl. Polym. Sci. 2014, 131, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef]
- Nianzhen, L.; Schwartz, M.; Ionescu-Zanetti, C. PDMS compound adsorption in context. J. Biomol. Screen. 2009, 14, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Van Midwoud, P.M.; Janse, A.; Merema, M.T.; Groothuis, G.M.M.; Verpoorte, E. Comparison of biocompatibility and adsorption properties of different plastics for advanced microfluidic cell and tissue culture models. Anal. Chem. 2012, 84, 3938–3944. [Google Scholar] [CrossRef]
- van Meer, B.J.; de Vries, H.; Firth, K.S.A.; van Weerd, J.; Tertoolen, L.G.J.; Karperien, H.B.J.; Jonkheijm, P.; Denning, C.; IJzerman, A.P.; Mummery, C.L. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Zant, E.; Grijpma, D.W. Tough biodegradable mixed-macromer networks and hydrogels by photo-crosslinking in solution. Acta Biomater. 2016, 31, 80–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuijer, R.; Bulstra, S.K.; Grijpma, D.W.; Feijen, J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials 2006, 27, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Geven, M.A.; Barbieri, D.; Yuan, H.; De Bruijn, J.D.; Grijpma, D.W. Preparation and mechanical properties of photo-crosslinked poly(trimethylene carbonate) and nano-hydroxyapatite composites. Clin. Hemorheol. Microcirc. 2015, 60, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zant, E.; Grijpma, D.W. Synthetic Biodegradable Hydrogels with Excellent Mechanical Properties and Good Cell Adhesion Characteristics Obtained by the Combinatorial Synthesis of Photo-Cross-Linked Networks. Biomacromolecules 2016, 17, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Chevtchik, N.V.; Fedecostante, M.; Jansen, J.; Mihajlovic, M.; Wilmer, M.; Rüth, M.; Masereeuw, R.; Stamatialis, D. Upscaling of a living membrane for bioartificial kidney device. Eur. J. Pharmacol. 2016, 790, 28–35. [Google Scholar] [CrossRef]
- Birket, M.J.; Ribeiro, M.C.; Kosmidis, G.; Ward, D.; Leitoguinho, A.R.; van de Pol, V.; Dambrot, C.; Devalla, H.D.; Davis, R.P.; Mastroberardino, P.G.; et al. Contractile Defect Caused by Mutation in MYBPC3 Revealed under Conditions Optimized for Human PSC-Cardiomyocyte Function. Cell Rep. 2015, 13, 733–745. [Google Scholar] [CrossRef] [Green Version]
- Pfizer Inc. Calan SR (verapamil hydrochloride) Sustained-Release Oral Caplets. LAB-02686-10.1, ID:4512000. 2019; pp. 1–14. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019152s041lbl.pdf (accessed on 25 March 2020).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development setting. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Buxboim, A.; Rajagopal, K.; Brown, A.E.X.; Discher, D.E. How deeply cells feel: Methods for thin gels. J. Phys. Condens. Matter 2010, 22, 194116. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [Green Version]
- Dalsin, J.L.; Messersmith, P.B. Bioinspired antifouling polymers. Mater. Today 2005, 8, 38–46. [Google Scholar] [CrossRef]
- Grossman, W.; Paulus, W.J. Myocardial stress and hypertrophy: A complex interface between biophysics and cardiac remodeling. J. Clin. Investig. 2013, 123, 3701–3703. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.; Raskin, A.; Chu, P.-H.; Lange, S.; Domenighetti, A.A.; Zheng, M.; Liang, X.; Zhang, T.; Yajima, T.; Gu, Y.; et al. An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J. Clin. Investig. 2008, 118, 3870–3880. [Google Scholar] [CrossRef] [PubMed]
- Oudit, G.Y.; Kassiri, Z.; Zhou, J.; Liu, Q.C.; Liu, P.P.; Backx, P.H.; Dawood, F.; Crackower, M.A.; Scholey, J.W.; Penninger, J.M. Loss of PTEN attenuates the development of pathological hypertrophy and heart failure in response to biomechanical stress. Cardiovasc. Res. 2008, 78, 505–514. [Google Scholar] [CrossRef]
- Pandey, P.; Hawkes, W.; Hu, J.; Megone, W.V.; Gautrot, J.; Anilkumar, N.; Zhang, M.; Hirvonen, L.; Cox, S.; Ehler, E.; et al. Cardiomyocytes Sense Matrix Rigidity through a Combination of Muscle and Non-muscle Myosin Contractions. Dev. Cell 2018, 44, 326–336.e3. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Lee, H.; Bray, M.A.; Geisse, N.A.; Huang, Y.T.; Adams, W.J.; Sheehy, S.P.; Parker, K.K. Myocyte shape regulates lateral registry of sarcomeres and contractility. Am. J. Pathol. 2012, 181, 2030–2037. [Google Scholar] [CrossRef] [Green Version]
- McCain, M.L.; Yuan, H.; Pasqualini, F.S.; Campbell, P.H.; Parker, K.K. Matrix elasticity regulates the optimal cardiac myocyte shape for contractility. AJP Heart Circ. Physiol. 2014, 306, H1525–H1539. [Google Scholar] [CrossRef] [PubMed]
- Decker, M.L.; Behnke-Barclay, M.; Cook, M.G.; La Pres, J.J.; Clark, W.A.; Decker, R.S. Cell shape and organization of the contractile apparatus in cultured adult cardiac myocytes. J. Mol. Cell. Cardiol. 1991, 23, 817–832. [Google Scholar] [CrossRef]
- Bray, M.A.; Sheehy, S.P.; Parker, K.K. Sarcomere alignment is regulated by myocyte shape. Cell Motil. Cytoskeleton 2008, 65, 641–651. [Google Scholar] [CrossRef] [Green Version]
- Parker, K.K.; Tan, J.; Chen, C.S.; Tung, L. Myofibrillar architecture in engineered cardiac myocytes. Circ. Res. 2008, 103, 340–342. [Google Scholar] [CrossRef]
- Sharma, A.; McKeithan, W.L.; Serrano, R.; Kitani, T.; Burridge, P.W.; del Álamo, J.C.; Mercola, M.; Wu, J.C. Use of human induced pluripotent stem cell–derived cardiomyocytes to assess drug cardiotoxicity. Nat. Protoc. 2018, 13, 3018–3041. [Google Scholar] [CrossRef]
- Savoji, H.; Mohammadi, M.H.; Rafatian, N.; Toroghi, M.K.; Wang, E.Y.; Zhao, Y.; Korolj, A.; Ahadian, S.; Radisic, M. Cardiovascular disease models: A game changing paradigm in drug discovery and screening. Biomaterials 2019, 198, 3–26. [Google Scholar] [CrossRef] [PubMed]




| Macromer | TMC Conversion (%) | df (%) | Mn (kg/mol) | Molar Ratio PTMC:PEG | |
|---|---|---|---|---|---|
| PTMC | PEG | ||||
| PTMC-dMA | 99.1 | 91.6 | 9.5 | - | - |
| PEG-dMA | - | 93.5 | - | 10.0 | - |
| PTMC-PEG-PTMC-dMA | 97.4 | 94.8 | 10.1 | 10.0 | 50:50 |
| Membrane Identifiers | Network Composition | Molar Ratio | Reactive Groups | Gel Content | |||
|---|---|---|---|---|---|---|---|
| # | Name | macromer 1 (mm1) | macromer 2 (mm2) | mm1:mm2 | PTMC:PEG | mol MA/g sol | (%) |
| M1 | PTMC | PTMC-dMA | - | - | - | 4.8 × 10−5 | 94 ± 1 |
| M2 | PEG | PEG-dMA | - | - | - | 4.6 × 10−5 | 96 ± 2 |
| M3 | PPP | PTMC-PEG-PTMC-dMA | - | - | 50:50 | 3.7 × 10−5 | 84 ± 1 |
| M4 | PTMC50:PEG50 | PTMC-dMA | PEG-dMA | 50:50 | 50:50 | 4.8 × 10−5 | 90 ± 4 |
| M5 | PTMC10:PEG90 | PTMC-dMA | PEG-dMA | 10:90 | 10:90 | 4.7 × 10−5 | 93 ± 1 |
| M6 | PPP26:PEG74 | PTMC-PEG-PTMC-dMA | PEG-dMA | 26:74 | 13:87 | 4.4 × 10−5 | 88 ± 2 |
| Membrane | Buffer Uptake | Young’s Modulus | dL at Break | Max Stress | Toughness | |
|---|---|---|---|---|---|---|
| # | Name | (%) | (kPa) | (%) | (kPa) | (N/mm2) |
| M1 | PTMC | 0.8 ± 0.3 | 2531 ± 120 | 1969 ± 253 | 2312 ± 335 | 2194 ± 423 |
| M2 | PEG | 2553 ± 257 | 18 ± 2 | 145 ± 23 | 14 ± 1 | 1.2 ± 0.3 |
| M3 | PPP | 319 ± 8 | 381 ± 14 | 911 ± 113 | 431 ± 50 | 370 ± 55 |
| M4 | PTMC50:PEG50 | 351 ± 9 | 338 ± 47 | 227 ± 50 | 286 ± 46 | 27 ± 6 |
| M5 | PTMC10:PEG90 | 1240 ± 21 | 37 ± 3 | 222 ± 44 | 33 ± 3 | 4 ± 1 |
| M6 | PPP26:PEG74 | 1053 ± 53 | 61 ± 6 | 109 ± 11 | 54 ± 7 | 4 ± 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allijn, I.; Ribeiro, M.; Poot, A.; Passier, R.; Stamatialis, D. Membranes for Modelling Cardiac Tissue Stiffness In Vitro Based on Poly(trimethylene carbonate) and Poly(ethylene glycol) Polymers. Membranes 2020, 10, 274. https://doi.org/10.3390/membranes10100274
Allijn I, Ribeiro M, Poot A, Passier R, Stamatialis D. Membranes for Modelling Cardiac Tissue Stiffness In Vitro Based on Poly(trimethylene carbonate) and Poly(ethylene glycol) Polymers. Membranes. 2020; 10(10):274. https://doi.org/10.3390/membranes10100274
Chicago/Turabian StyleAllijn, Iris, Marcelo Ribeiro, André Poot, Robert Passier, and Dimitrios Stamatialis. 2020. "Membranes for Modelling Cardiac Tissue Stiffness In Vitro Based on Poly(trimethylene carbonate) and Poly(ethylene glycol) Polymers" Membranes 10, no. 10: 274. https://doi.org/10.3390/membranes10100274