Sustained Liver HBsAg Loss and Clonal T- and B-Cell Expansion upon Therapeutic DNA Vaccination Require Low HBsAg Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
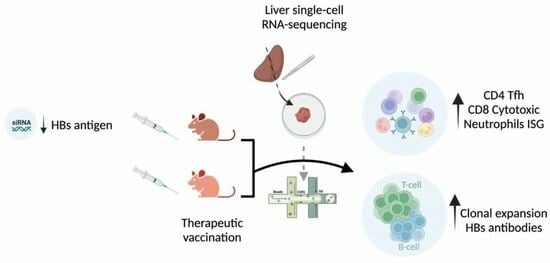
2.2. Animal Models and Study Designs
2.3. Isolation of Intrahepatic Lymphocytes (IHLs)
2.4. Detection of HBV-Specific T Cells by ELISPOT
2.5. Viral Parameters and Alanine Aminotransferase (ALT) Analyses
2.6. Histology and Immunohistochemistry (IHC)
2.7. Image Analysis
2.8. Single-Cell RNA Sequencing and Data Analysis
2.9. Single-Cell V(D)J Analysis
2.10. Statistical Analysis
3. Results
3.1. Sequential Treatment with GalNAc-HBV siRNA and Therapeutic Vaccination Showed Sustained HBsAg Loss
3.2. Liver CD4 Tfh-like Cell Subpopulation Induced by Vaccination, as Identified by Single-Cell RNA Sequencing Analysis
3.3. Decrease in Liver Naïve CD8 T Cells and Increase in Pre-Exhausted Naïve Cells upon Vaccination
3.4. Macrophage, NK Cell and Neutrophil Compartments Change upon Therapeutic Vaccination
3.5. Therapeutic Vaccination Induces TCR Clonal Expansion across Multiple T-Cell Subtypes
3.6. BCR Clonal Expansion across Atypical B Cells and Plasma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Hepatitis B; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 26 November 2023).
- Werle-Lapostolle, B.; Bowden, S.; Locarnini, S.; Wursthorn, K.; Petersen, J.; Lau, G.; Trepo, C.; Marcellin, P.; Goodman, Z.; Delaney, W.E., IV; et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 2004, 126, 1750–1758. [Google Scholar] [CrossRef]
- Zoulim, F.; Locarnini, S. Hepatitis B Virus Resistance to Nucleos(t)ide Analogues. Gastroenterology 2009, 137, 1593–1608.e2. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- A Revill, P.; Chisari, F.V.; Block, J.M.; Dandri, M.; Gehring, A.J.; Guo, H.; Hu, J.; Kramvis, A.; Lampertico, P.; A Janssen, H.L.; et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol. Hepatol. 2019, 4, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; Zoulim, F.; Dusheiko, G.; Ghany, M.G. Hepatitis B cure: From discovery to regulatory approval. Hepatology 2017, 66, 1296–1313. [Google Scholar] [CrossRef] [PubMed]
- Rehermann, B. Pathogenesis of chronic viral hepatitis: Differential roles of T cells and NK cells. Nat. Med. 2013, 19, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-J.; Wong, D.K.; Wahed, A.S.; Lee, W.M.; Feld, J.J.; Terrault, N.; Khalili, M.; Sterling, R.K.; Kowdley, K.V.; Bzowej, N.; et al. Hepatitis B Virus–Specific and Global T-Cell Dysfunction in Chronic Hepatitis B. Gastroenterology 2016, 150, 684–695.e685. [Google Scholar] [CrossRef] [PubMed]
- Thimme, R.; Wieland, S.; Steiger, C.; Ghrayeb, J.; Reimann, K.A.; Purcell, R.H.; Chisari, F.V. CD8+T Cells Mediate Viral Clearance and Disease Pathogenesis during Acute Hepatitis B Virus Infection. J. Virol. 2003, 77, 68–76. [Google Scholar] [CrossRef]
- Yang, S.; Zeng, W.; Zhang, J.; Lu, F.; Chang, J.; Guo, J.-T. Restoration of a functional antiviral immune response to chronic HBV infection by reducing viral antigen load: If not sufficient, is it necessary? Emerg. Microbes Infect. 2021, 10, 1545–1554. [Google Scholar] [CrossRef]
- Gerlich, W.H. Medical Virology of Hepatitis B: How it began and where we are now. Virol. J. 2013, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Cargill, T.; Barnes, E. Therapeutic vaccination for treatment of chronic hepatitis B. Clin. Exp. Immunol. 2021, 205, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Michler, T.; Kosinska, A.D.; Festag, J.; Bunse, T.; Su, J.; Ringelhan, M.; Imhof, H.; Grimm, D.; Steiger, K.; Mogler, C.; et al. Knockdown of Virus Antigen Expression Increases Therapeutic Vaccine Efficacy in High-Titer Hepatitis B Virus Carrier Mice. Gastroenterology 2020, 158, 1762–1775.e9. [Google Scholar] [CrossRef] [PubMed]
- T Evans, L.; Bussey, S.L.; Teo, A.; Tria, A.; Brown, R.; Mehta, K.; Anderson, A.; Vardeu, W.L.; Chuang, C.C.; Yi, Y.S.; et al. Phase 1b/2a study of heterologous ChAdOx1-HBV/MVA-HBV therapeutic vaccination (VTP-300) as monotherapy and combined with low-dose nivolumab in virally-suppressed patients with CHB on nucleos(t)ide analogues. J. Hepatol. 2023, 9, 30. [Google Scholar]
- Genshaft, A.S.; Subudhi, S.; Keo, A.; Vasquez, J.D.S.; Conceição-Neto, N.; Mahamed, D.; Boeijen, L.L.; Alatrakchi, N.; Oetheimer, C.; Vilme, M.; et al. Single-cell RNA sequencing of liver fine-needle aspirates captures immune diversity in the blood and liver in chronic hepatitis B patients. Hepatology 2023, 78, 1525–1541. [Google Scholar] [CrossRef] [PubMed]
- Nkongolo, S.; Mahamed, D.; Kuipery, A.; Vasquez, J.D.S.; Kim, S.C.; Mehrotra, A.; Patel, A.; Hu, C.; McGilvray, I.; Feld, J.J.; et al. Longitudinal liver sampling in patients with chronic hepatitis B starting antiviral therapy reveals hepatotoxic CD8+ T cells. J. Clin. Investig. 2023, 133, e158903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Cheng, Y.; Meng, F.; Song, J.-W.; Fan, X.; Fan, H.; Li, J.; Fu, Y.-L.; Zhou, M.-J.; et al. Single-cell RNA sequencing reveals intrahepatic and peripheral immune characteristics related to disease phases in HBV-infected patients. Gut 2023, 72, 153–167. [Google Scholar] [CrossRef]
- Zhu, R.; Han, Q.; Neto, N.; Yao, Z.; Wu, Q.; de Maeyer, D.; Van der Borght, K.; Beyens, M.; Van Gulck, E.; Kukolj, G.; et al. AAV-HBV mouse model replicates immune exhaustion patterns of chronic HBV patients at single-cell level. J. Hepatol. 2023, 78, S443. [Google Scholar] [CrossRef]
- De Pooter, D.; Van Gulck, E.; Chen, A.; Evans, C.F.; Neefs, J.-M.; Horton, H.; Boden, D. A Therapeutic Hepatitis B Virus DNA Vaccine Induces Specific Immune Responses in Mice and Non-Human Primates. Vaccines 2021, 9, 969. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M., 3rd; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Miao, V.N.; Owings, A.H.; Navia, A.W.; Tang, Y.; Bromley, J.D.; Lotfy, P.; Sloan, M.; Laird, H.; Williams, H.B.; et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell 2021, 184, 4713–4733.e22. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Bonnardel, J.; Haest, B.; Vanderborght, B.; Wagner, C.; Remmerie, A.; Bujko, A.; Martens, L.; Thoné, T.; Browaeys, R.; et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell 2022, 185, 379–396.e38. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.; Szabo, T.; Fotakis, G.; Haider, M.; Rieder, D.; Trajanoski, Z.; Finotello, F. Scirpy: A Scanpy extension for analyzing single-cell T-cell receptor-sequencing data. Bioinformatics 2020, 36, 4817–4818. [Google Scholar] [CrossRef] [PubMed]
- Suo, C.; Polanski, K.; Dann, E.; Lindeboom, R.G.H.; Vilarrasa-Blasi, R.; Vento-Tormo, R.; Haniffa, M.; Meyer, K.B.; Dratva, L.M.; Tuong, Z.K.; et al. Dandelion uses the single-cell adaptive immune receptor repertoire to explore lymphocyte developmental origins. Nat. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Giudicelli, V.; Chaume, D.; Lefranc, M.-P. IMGT/GENE-DB: A comprehensive database for human and mouse immunoglobulin and T cell receptor genes. Nucleic Acids Res. 2004, 33, D256–D261. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.T.; Heiden, J.A.V.; Uduman, M.; Gadala-Maria, D.; Yaari, G.; Kleinstein, S.H. Change-O: A toolkit for analyzing large-scale B cell immunoglobulin repertoire sequencing data. Bioinformatics 2015, 31, 3356–3358. [Google Scholar] [CrossRef] [PubMed]
- Heiden, J.A.V.; Yaari, G.; Uduman, M.; Stern, J.N.; O’connor, K.C.; Hafler, D.A.; Vigneault, F.; Kleinstein, S.H. pRESTO: A toolkit for processing high-throughput sequencing raw reads of lymphocyte receptor repertoires. Bioinformatics 2014, 30, 1930–1932. [Google Scholar] [CrossRef]
- Zehn, D.; Thimme, R.; Lugli, E.; de Almeida, G.P.; Oxenius, A. ‘Stem-like’ precursors are the fount to sustain persistent CD8(+) T cell responses. Nat. Immunol. 2022, 23, 836–847. [Google Scholar] [CrossRef]
- Yao, C.; Sun, H.-W.; Lacey, N.E.; Ji, Y.; Moseman, E.A.; Shih, H.-Y.; Heuston, E.F.; Kirby, M.; Anderson, S.; Cheng, J.; et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8+ T cell persistence in chronic infection. Nat. Immunol. 2019, 20, 890–901. [Google Scholar] [CrossRef]
- Liu, X.; Yao, J.; Zhao, Y.; Wang, J.; Qi, H. Heterogeneous plasma cells and long-lived subsets in response to immunization, autoantigen and microbiota. Nat. Immunol. 2022, 23, 1564–1576. [Google Scholar] [CrossRef]
- Zheng, J.R.; Wang, Z.L.; Feng, B. Hepatitis B functional cure and immune response. Front. Immunol. 2022, 13, 1075916. [Google Scholar] [CrossRef]
- Cai, Y.; Yin, W. The Multiple Functions of B Cells in Chronic HBV Infection. Front. Immunol. 2020, 11, 582292. [Google Scholar] [CrossRef]
- Su, J.; Brunner, L.; Oz, E.A.; Sacherl, J.; Frank, G.; Kerth, H.A.; Thiele, F.; Wiegand, M.; Mogler, C.; Aguilar, J.C.; et al. Activation of CD4 T cells during prime immunization determines the success of a therapeutic hepatitis B vaccine in HBV-carrier mouse models. J. Hepatol. 2023, 78, 717–730. [Google Scholar] [CrossRef]
- Gane, E.; Verdon, D.J.; Brooks, A.E.; Gaggar, A.; Nguyen, A.H.; Subramanian, G.M.; Schwabe, C.; Dunbar, P.R. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J. Hepatol. 2019, 71, 900–907. [Google Scholar] [CrossRef]
- Zahid, K.R.; Raza, U.; Tumbath, S.; Jiang, L.; Xu, W.; Huang, X. Neutrophils: Musketeers against immunotherapy. Front. Oncol. 2022, 12, 975981. [Google Scholar] [CrossRef]
- Hu, S.; Liu, X.; Gao, Y.; Zhou, R.; Wei, M.; Dong, J.; Yan, H.; Zhao, Y. Hepatitis B Virus Inhibits Neutrophil Extracellular Trap Release by Modulating Reactive Oxygen Species Production and Autophagy. J. Immunol. 2019, 202, 805–815. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conceição-Neto, N.; Pierson, W.; Vacca, M.; Beyens, M.; De Clerck, B.; Aerts, L.; Voeten, B.; De Pooter, D.; Verschueren, L.; Dockx, K.; et al. Sustained Liver HBsAg Loss and Clonal T- and B-Cell Expansion upon Therapeutic DNA Vaccination Require Low HBsAg Levels. Vaccines 2023, 11, 1825. https://doi.org/10.3390/vaccines11121825
Conceição-Neto N, Pierson W, Vacca M, Beyens M, De Clerck B, Aerts L, Voeten B, De Pooter D, Verschueren L, Dockx K, et al. Sustained Liver HBsAg Loss and Clonal T- and B-Cell Expansion upon Therapeutic DNA Vaccination Require Low HBsAg Levels. Vaccines. 2023; 11(12):1825. https://doi.org/10.3390/vaccines11121825
Chicago/Turabian StyleConceição-Neto, Nádia, Wim Pierson, Maurizio Vacca, Matthias Beyens, Ben De Clerck, Liese Aerts, Birgit Voeten, Dorien De Pooter, Lore Verschueren, Koen Dockx, and et al. 2023. "Sustained Liver HBsAg Loss and Clonal T- and B-Cell Expansion upon Therapeutic DNA Vaccination Require Low HBsAg Levels" Vaccines 11, no. 12: 1825. https://doi.org/10.3390/vaccines11121825