Exploring Seminal Plasma GSTM3 as a Quality and In Vivo Fertility Biomarker in Pigs—Relationship with Sperm Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Samples
2.3. Experimental Design
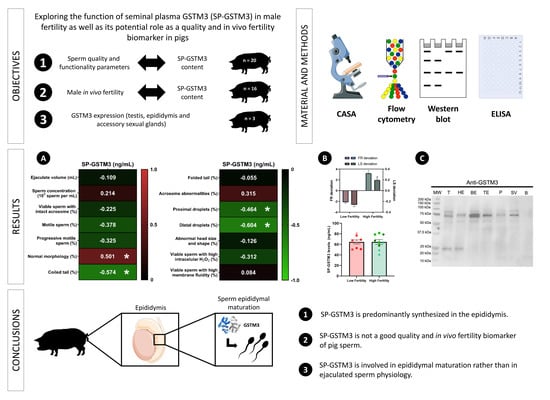
2.3.1. Relationship Between SP-GSTM3 Concentration and Sperm Quality and Functionality Parameters
2.3.2. Expression of GSTM3 in Boar Testis, Epididymis and Accessory Sexual Glands
2.3.3. Relationship Between SP-GSTM3 Content and In Vivo Fertility of Liquid-Stored Semen Samples
2.4. Sperm Quality and Functionality Assessment
2.5. Western Blot Analysis
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Statistical Analysis
3. Results
3.1. Characterisation of Porcine SP-GSTM3
3.2. Correlation between SP-GSTM3 and Sperm Quality and Functionality Parameters of Semen Samples
3.3. Relationship between SP-GSTM3 Concentration and Sperm Resilience to Withstand Liquid-Storage at 17 °C
3.4. Presence of GSTM3 in SP-Related Testis and Accessory Sexual Glands
3.5. Relationship between SP-GSTM3 and In Vivo Fertility Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial insemination |
| SP | Seminal plasma |
| BE | Corpus epididymis |
| BE | Bulbourethral glands |
| CM-H2DCFDA | 5- and 6-chloromethyl-2, 7-dichlorodihydrofluorescein diacetate acetyl ester |
| DMSO | Dymetil sulfoxide |
| ELISA | Enzyme-linked immunosorbent assay |
| FITC | Fluorescein isothiocyanate |
| FR | Farrowing rate |
| GPX5 | Glutathione peroxidase 5 |
| GSTM3 | Glutathione S-transferase Mu 3 |
| GSTs | Glutathione S-transferases |
| H-42 | Hoechst 33342 |
| HE | Caput epididymis |
| LS | Litter size |
| M540 | Merocyanine 540 |
| P | Prostate |
| PI | Propidium iodide |
| PNA | Peanut agglutinin |
| PNA | Peanut agglutinin |
| PON1 | Paraoxonase 1 |
| PVDF | Polyvinylidene fluoride |
| RT | Room temperature |
References
- Waberski, D.; Riesenbeck, A.; Schulze, M.; Weitze, K.F.; Johnson, L. Application of preserved boar semen for artificial insemination: Past, present and future challenges. Theriogenology 2019, 137, 2–7. [Google Scholar] [CrossRef]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M.C. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Yeste, M. State-of-the-art of boar sperm preservation in liquid and frozen state. Anim. Reprod. 2017, 14, 69–81. [Google Scholar] [CrossRef]
- Roca, J.; Broekhuijse, M.; Parrilla, I.; Rodriguez-Martinez, H.; Martinez, E.A.; Bolarin, A. Boar Differences In Artificial Insemination Outcomes: Can They Be Minimized? Reprod. Domest. Anim. 2015, 50, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Roca, J.; Parrilla, I.; Bolarin, A.; Martinez, E.A.; Rodriguez-Martinez, H. Will AI in pigs become more efficient? Theriogenology 2016, 86, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonet, S.; Garcia, E.; Sepúlveda, L. The Boar Reproductive System. In Boar Reproduction: Fundamentals and New Biotechnological Trends; Springer: Heidelberg/Berlin, Germany, 2013; pp. 65–108. [Google Scholar]
- Sancho, S.; Vilagran, I. The Boar Ejaculate: Sperm Function and Seminal Plasma Analyses. In Boar Reproduction: Fundamentals and New Biotechnological Trends; Springer: Heidelberg/Berlin, Germany, 2013; pp. 471–516. [Google Scholar]
- Rodríguez-Martínez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal plasma proteins: What role do they play? Am. J. Reprod. Immunol. 2011, 66, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Holland, A.; Ohlendieck, K. Comparative profiling of the sperm proteome. Proteomics 2015, 15, 632–648. [Google Scholar] [CrossRef]
- González-Cadavid, V.; Martins, J.A.M.; Moreno, F.B.; Andrade, T.S.; Santos, A.C.L.; Monteiro-Moreira, A.C.O.; Moreira, R.A.; Moura, A.A. Seminal plasma proteins of adult boars and correlations with sperm parameters. Theriogenology 2014, 82, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Patiño, C.; Barranco, I.; Parrilla, I.; Valero, M.L.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Characterization of the porcine seminal plasma proteome comparing ejaculate portions. J. Proteomics 2016, 142, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Patiño, C.; Parrilla, I.; Barranco, I.; Vergara-Barberán, M.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M.; Rodriguez-Martínez, H.; Martínez, E.A.; Roca, J. New In-Depth Analytical Approach of the Porcine Seminal Plasma Proteome Reveals Potential Fertility Biomarkers. J. Proteome Res. 2018, 17, 1065–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lazari, F.L.; Sontag, E.R.; Schneider, A.; Araripe Moura, A.A.; Vasconcelos, F.R.; Nagano, C.S.; Dalberto, P.F.; Bizarro, C.V.; Mattos, R.C.; Mascarenhas Jobim, M.I.; et al. Proteomic identification of boar seminal plasma proteins related to sperm resistance to cooling at 17 °C. Theriogenology 2020, 147, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Barranco, S.; Tvarijonaviciute, A.; Perez-Patiho, C.; Vicente-Carrillo, A.; Parrilla, N.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Glutathione peroxidase 5 is expressed by the entire pig male genital tract and once in the seminal plasma contributes to sperm survival and in vivo fertility. PLoS ONE 2016, 11, e0162958. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patiño, C.; Alkmin, D.V.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. The activity of paraoxonase type 1 (PON-1) in boar seminal plasma and its relationship with sperm quality, functionality and in vivo fertility. Andrology 2015, 3, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Barranco, I.; Tvarijonaviciute, A.; Molina, M.F.; Martinez, E.A.; Rodriguez-Martinez, H.; Parrilla, I.; Roca, J. Seminal plasma antioxidants are directly involved in boar sperm cryotolerance. Theriogenology 2018, 107, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llavanera, M.; Mateo-Otero, Y.; Bonet, S.; Barranco, I.; Fernández-Fuertes, B.; Yeste, M. The triple role of glutathione S-transferases in mammalian male fertility. Cell. Mol. Life Sci. 2019, 77, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Llavanera, M.; Delgado-bermúdez, A.; Olives, S.; Mateo-otero, Y.; Recuero, S.; Bonet, S.; Fernández-Fuertes, B.; Yeste, M.; Barranco, I. Glutathione S-transferases play a crucial role in mitochondrial function, plasma membrane stability and oxidative regulation of mammalian sperm. Antioxidants 2020, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Hemachand, T.; Shaha, C. Functional role of sperm surface glutathione S-transferases and extracellular glutathione in the haploid spermatozoa under oxidative stress. FEBS Lett. 2003, 538, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Aydos, S.E.; Taspinar, M.; Sunguroglu, A. Association of CYP1A1 and glutathione S-transferase polymorphisms with male factor infertility. Fertil. Steril. 2009, 92, 541–547. [Google Scholar] [CrossRef]
- Vani, G.T.; Mukesh, N.; Siva Prasad, B.; Rama Devi, P.; Hema Prasad, M.; Rani, P.U.; Reddy, P.P. Role of glutathione S-transferase Mu-1 (GSTM1) polymorphism in oligospermic infertile males. Andrologia 2010, 42, 213–217. [Google Scholar] [CrossRef]
- Kolesnikova, L.I.; Kurashova, N.A.; Bairova, T.A.; Dolgikh, M.I.; Ershova, O.A.; Dashiev, B.G.; Korytov, L.I.; Koroleva, N.V. Role of Glutathione-S-Transferase Family Genes in Male Infertility. Bull. Exp. Biol. Med. 2017, 163, 643–645. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Shafiei, N.; Safarinejad, S. The association of glutathione-S-transferase gene polymorphisms (GSTM1, GSTT1, GSTP1) with idiopathic male infertility. J. Hum. Genet. 2010, 55, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Lakpour, N.; Mirfeizollahi, A.; Farivar, S.; Akhondi, M.M.; Hashemi, S.B.; Amirjannati, N.; Heidari-Vala, H.; Sadeghi, M.R. The association of seminal plasma antioxidant levels and sperm chromatin status with genetic variants of GSTM1 and GSTP1 (Ile105Val and Ala114Val) in infertile men with oligoasthenoteratozoospermia. Dis. Markers 2013, 34, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wang, S.; Wang, W.; Cao, Q.; Qin, C.; Liu, B.; Li, P.; Zhang, W. The glutathione-S-transferase gene polymorphisms (GSTM1 and GSTT1) and idiopathic male infertility risk: A meta-analysis. Gene 2012, 511, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.P.; Wu, F.L.; Guo, W.B.; Wang, Y.Z.; Li, J.P.; Huang, Y.Q.; Li, S.G.; Liu, J.P. Null genotypes of GSTM1 and GSTT1 contribute to male factor infertility risk: A meta-analysis. Fertil. Steril. 2013, 99, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Y.; Cai, Q.; Zhang, Y.; Niu, Y. Association of the Glutathione S-transferases M1 and T1 polymorphism with male infertility: A meta-Analysis. J. Assist. Reprod. Genet. 2013, 30, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Aydemir, B.; Onaran, I.; Kiziler, A.R.; Alici, B.; Akyolcu, M.C. Increased oxidative damage of sperm and seminal plasma in men with idiopathic infertility is higher in patients with glutathione S-transferase Mu-1 null genotype. Asian J. Androl. 2007, 9, 108–115. [Google Scholar] [CrossRef]
- Kwon, W.S.; Oh, S.A.; Kim, Y.J.; Rahman, M.S.; Park, Y.J.; Pang, M.G. Proteomic approaches for profiling negative fertility markers in inferior boar spermatozoa. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Llavanera, M.; Delgado-Bermuúdez, A.; Fernandez-Fuertes, B.; Recuero, S.; Mateo, Y.; Bonet, S.; Barranco, I.; Yeste, M. GSTM3, but not IZUMO1, is a cryotolerance marker of boar sperm. J. Anim. Sci. Biotechnol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Broekhuijse, M.L.W.J.; Šoštarić, E.; Feitsma, H.; Gadella, B.M. The value of microscopic semen motility assessment at collection for a commercial artificial insemination center, a retrospective study on factors explaining variation in pig fertility. Theriogenology 2012, 77, 1466–1479. [Google Scholar] [CrossRef]
- Barranco, I.; Roca, J.; Tvarijonaviciute, A.; Rubér, M.; Vicente-Carrillo, A.; Atikuzzaman, M.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H. Measurement of activity and concentration of paraoxonase 1 (PON-1) in seminal plasma and identification of PON-2 in the sperm of boar ejaculates. Mol. Reprod. Dev. 2015, 82, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Smital, J.; De Sousa, L.L.; Mohsen, A. Differences among breeds and manifestation of heterosis in AI boar sperm output. Anim. Reprod. Sci. 2004, 80, 121–130. [Google Scholar] [CrossRef]
- Bonet, S.; Briz, M.D.; Yeste, M. A Proper Assessment of Boar Sperm Function May Not Only Require Conventional Analyses but Also Others Focused on Molecular Markers of Epididymal Maturation. Reprod. Domest. Anim. 2012, 47, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Briz, M.D.; Fàbrega, A. The boar spermatozoon. In Boar Reproduction: Fundamentals and New Biotechnological Trends; Springer: Heidelberg/Berlin, Germany, 2013; pp. 3–47. [Google Scholar]
- Cooper, T.G. Cytoplasmic droplets: The good, the bad or just confusing? Hum. Reprod. 2005, 20, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Briz, M.D.; Bonet, S.; Pinart, B.; Camps, R. Sperm malformations throughout the boar epididymal duct. Anim. Reprod. Sci. 1996, 43, 221–239. [Google Scholar] [CrossRef]
- Otasevic, V.; Kalezic, A.; Macanovic, B.; Jankovic, A.; Stancic, A.; Garalejic, E.; Korac, A.; Korac, B. Evaluation of the antioxidative enzymes in the seminal plasma of infertile men: Contribution to classic semen quality analysis. Syst. Biol. Reprod. Med. 2019, 65, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Macanovic, B.; Vucetic, M.; Jankovic, A.; Stancic, A.; Buzadzic, B.; Garalejic, E.; Korac, A.; Korac, B.; Otasevic, V. Correlation between sperm parameters and protein expression of antioxidative defense enzymes in seminal plasma: A pilot study. Dis. Markers 2015, 2015, 436236. [Google Scholar] [CrossRef]
- Barranco, I.; Padilla, L.; Tvarijonaviciute, A.; Parrilla, I.; Martínez, E.A.; Rodriguez-Martinez, H.; Yeste, M.; Roca, J. Levels of activity of superoxide dismutase in seminal plasma do not predict fertility of pig AI-semen doses. Theriogenology 2019, 140, 18–24. [Google Scholar] [CrossRef]





| Sperm Quality and Functionality Parameters | Mean ± SEM | Range (Min–Max) |
|---|---|---|
| Ejaculate volume (mL) | 619.05 ± 21.28 | 357–729 |
| Sperm concentration (106 sperm per mL) | 171.93 ± 10.85 | 91.65–256 |
| Viable sperm with intact acrosome (%) | 84.73 ± 1.47 | 72.10–91.60 |
| Motile sperm (%) | 76.85 ± 2.03 | 51–90 |
| Progressive motile sperm (%) | 50 ± 2.36 | 26–66 |
| Normal morphology (%) | 77.95 ± 3.14 | 40–95 |
| Coiled tails (%) | 0.30 ± 0.13 | 0–2 |
| Folded tails (%) | 6.25 ± 1.26 | 0–19 |
| Acrosome abnormalities (%) | 3.32 ± 0.95 | 0–17 |
| Proximal droplets (%) | 6 ± 1.55 | 0–26 |
| Distal droplets (%) | 5.45 ± 1.57 | 0–29 |
| Abnormal head size and shape (%) | 0.90 ± 0.35 | 0–5 |
| Viable sperm with high intracellular H2O2 (%) | 30.69 ± 3.58 | 3.40–56.40 |
| Viable sperm with high plasma membrane fluidity (%) | 1.69 ± 0.19 | 0.50–3.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llavanera, M.; Delgado-Bermúdez, A.; Mateo-Otero, Y.; Padilla, L.; Romeu, X.; Roca, J.; Barranco, I.; Yeste, M. Exploring Seminal Plasma GSTM3 as a Quality and In Vivo Fertility Biomarker in Pigs—Relationship with Sperm Morphology. Antioxidants 2020, 9, 741. https://doi.org/10.3390/antiox9080741
Llavanera M, Delgado-Bermúdez A, Mateo-Otero Y, Padilla L, Romeu X, Roca J, Barranco I, Yeste M. Exploring Seminal Plasma GSTM3 as a Quality and In Vivo Fertility Biomarker in Pigs—Relationship with Sperm Morphology. Antioxidants. 2020; 9(8):741. https://doi.org/10.3390/antiox9080741
Chicago/Turabian StyleLlavanera, Marc, Ariadna Delgado-Bermúdez, Yentel Mateo-Otero, Lorena Padilla, Xavier Romeu, Jordi Roca, Isabel Barranco, and Marc Yeste. 2020. "Exploring Seminal Plasma GSTM3 as a Quality and In Vivo Fertility Biomarker in Pigs—Relationship with Sperm Morphology" Antioxidants 9, no. 8: 741. https://doi.org/10.3390/antiox9080741