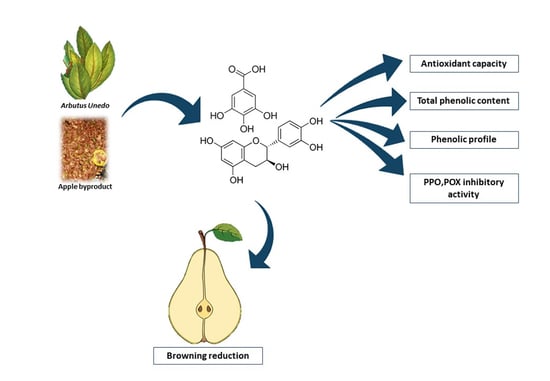
Natural-Based Antioxidant Extracts as Potential Mitigators of Fruit Browning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Extraction Procedure
2.4. Total Phenolic Content (TPC)
2.5. Antioxidant Activity by ABTS Radical Scavenging Activity Assay
2.6. In Vitro Polyphenoloxidase and Peroxidase Inhibition Assay
2.7. Preparation and Treatment of Pear Disc
2.8. Identification of Phenolic Compounds by LC-ESI-UHR-QqTOF-HRMS
2.9. Quantification of Phenolic Compounds by HPLC-PDA
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of NES
3.1.1. Total Phenolic Content
3.1.2. Antioxidant Activity
3.1.3. Characterization of Phenolic Compounds Composition of NES
3.2. Inhibitory Effect of the Selected Extracts on Browning Enzymes Acitivity
3.3. Efficacy of the Natural Extracts in Inhibiting Browning in Pear Cut Discs
3.4. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gatto, M.A.; Sergio, L.; Ippolito, A.; Di Venere, D. Phenolic extracts from wild edible plants to control postharvest diseases of sweet cherry fruit. Postharvest Biol. Technol. 2016, 120, 180–187. [Google Scholar] [CrossRef]
- Ioannou, I.; Ghoul, M. Prevention of enzymatic browning in fruit and vegetables. Eur. Sci. J. 2013, 9, 57–81. [Google Scholar]
- Singh, B.; Suri, K.; Shevkani, K.; Kaur, A.; Kaur, A.; Singh, N. Enzymatic browning of fruit and vegetables: A review. In Enzymes in Food Technology; Springer: Singapore, 2018; pp. 63–78. [Google Scholar]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Identification and characterization of serotonin as an anti-browning compound of apple and pear. Postharvest Biol. Technol. 2015, 110, 183–189. [Google Scholar] [CrossRef]
- Kaushik, P.; Gramazio, P.; Vilanova, S.; Raigón, M.D.; Prohens, J.; Plazas, M. Phenolics content, fruit flesh colour and browning in cultivated eggplant, wild relatives and interspecific hybrids and implications for fruit quality breeding. Food Res. Int. 2017, 102, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Jou, A.F.J.; Chen, S.H.; Tien, C.Y.; Cheng, C.F.; Fan, N.C.; Ho, J.A.A. Antioxidant, anti-inflammatory and anti-browning activities of hot water extracts of oriental herbal teas. Food Funct. 2010, 1, 200–208. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Z.; Xu, X.; Wang, Q.; Qin, G.; Tian, S. Crucial roles of membrane stability and its related proteins in the tolerance of peach fruit to chilling injury. Amino Acids 2010, 39, 181–194. [Google Scholar] [CrossRef]
- Dias, C.; Amaro, A.L.; Salvador, Â.C.; Silvestre, A.J.D.; Rocha, S.M.; Isidoro, N.; Pintado, M. Strategies to preserve postharvest quality of horticultural crops and superficial scald control: From diphenylamine antioxidant usage to more recent approaches. Antioxidants 2020, 9, 356. [Google Scholar] [CrossRef]
- He, Q.; Luo, Y.; Chen, P. Elucidation of the mechanism of enzymatic browning inhibition by sodium chlorite. Food Chem. 2008, 110, 847–851. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, C.Y.; Park, I. Prevention of enzymatic browning of pear by onion extract. Food Chem. 2005, 89, 181–184. [Google Scholar] [CrossRef]
- Jang, M.S.; Sanada, A.; Ushio, H.; Tanaka, M.; Ohshima, T. Inhibitory effects of “enokitake” mushroom extracts on polyphenol oxidase and prevention of apple browning. Lwt Food Sci. Technol. 2002, 35, 697–702. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi, S.; Jayalakshmi, S.K.; Sreeramulu, K. Polyphenols from different agricultural residues: Extraction, identification and their antioxidant properties. J. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K. Therapeutic and nutraceutical potential of bioactive compounds extracted from fruit residues. Crit. Rev. Food Sci. Nutr. 2015, 55, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Oliveira, A.; Coelho, M.; Alexandre, E.M.C.; Gomes, M.H.; Almeida, D.P.F.; Pintado, M. Effect of modified atmosphere on phytochemical profile of pasteurized peach purées. Lwt Food Sci. Technol. 2015, 64, 520–527. [Google Scholar] [CrossRef]
- Galeazzi, M.A.M.; Sgarbieri, V.C. Substrate specificity and Inhibition of Polyphenoloxidase (PPO) from a dwarf variety of banana (Muss Cavendishii, L.). J. Food Sci. 1981, 46, 1404–1406. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and Ozone-Induced Biochemical Changes in Antioxidant Enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Monforte, A.R.; Martins, S.I.F.S.; Silva Ferreira, A.C. Strecker aldehyde formation in wine: New insights into the role of gallic acid, glucose, and metals in phenylacetaldehyde formation. J. Agric. Food Chem. 2018, 66, 2459–2466. [Google Scholar] [CrossRef]
- Vilas-boas, A.A.; Oliveira, A.; Jesus, D.; Rodrigues, C.; Figueira, C.; Gomes, A. Chlorogenic acids composition and the impact of in vitro gastrointestinal digestion on espresso co ff ee from single-dose capsule. Food Res. Int. 2020, 134, 109223. [Google Scholar] [CrossRef]
- Karikas, G.A. Iridoids from Arbutus unedo. Fitoterapia 1993, 64, 187–193. [Google Scholar]
- Males, Z.; Plazibat, M.; Vundać, V.B.; Zuntar, I. Qualitative and quantitative analysis of flavonoids of the strawberry tree—Arbutus unedo L. (Ericaceae). Acta Pharm. 2006, 56, 245–250. [Google Scholar] [PubMed]
- Nara, K.; Miyoshi, T.; Honma, T.; Koga, H. Antioxidative activity of bound-form phenolics in potato peel. Biosci. Biotechnol. Biochem. 2014, 70, 1489–1491. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Industrial Crops & Products Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crop. Prod. 2018, 122, 498–505. [Google Scholar]
- Malheiro, R.; Sá, O.; Pereira, E.; Aguiar, C.; Baptista, P.; Pereira, J.A. Arbutus unedo L. leaves as source of phytochemicals with bioactive properties. Ind. Crop. Prod. 2012, 37, 473–478. [Google Scholar] [CrossRef]
- Moualek, I.; Aiche, G.I.; Guechaoui, N.M.; Lahcene, S.; Houali, K. Antioxidant and anti-inflammatory activities of Arbutus unedo aqueous extract. Asian Pac. J. Trop. Biomed. 2016, 6, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Erkekoglou, I.; Nenadis, N.; Samara, E.; Mantzouridou, F.T. Functional teas from the leaves of arbutus unedo: Phenolic content, antioxidant activity, and detection of efficient radical scavengers. Plant Foods Hum. Nutr. 2017, 72, 176–183. [Google Scholar] [CrossRef]
- Oliveira, I.; Coelho, V.; Baltasar, R.; Pereira, J.A.; Baptista, P. Scavenging capacity of strawberry tree (Arbutus unedo L.) leaves on free radicals. Food Chem. Toxicol. 2009, 47, 1507–1511. [Google Scholar] [CrossRef]
- Bouyahya, A.; Moussaoui, N.; Abrini, J.; Bakri, Y.; Dakka, N. Determination of phenolic contents, antioxidant and antibacterial activities of strawberry tree (Arbutus unedo L.) leaf extracts. Br. Biotechnol. J. 2016, 14, 1–10. [Google Scholar] [CrossRef]
- Pavlovic, R.; Lakusic, B.; Doslov-Kokorus, Z.; Kovacevic, N. Arbutin content and antioxidant activity of some Ericaceae species Arbutin content and antioxidant activity of some Ericaceae species. Pharmazie 2009, 64, 656–659. [Google Scholar]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of flavonoids and iridoids to the hypoglycaemic, antioxidant, and nitric oxide (NO) inhibitory activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobaleda-Velasco, M.; Alanis-Bañuelos, R.E.; Almaraz-Abarca, N.; Rojas-López, M.; González-Valdez, L.S.; Ávila-Reyes, J.A.; Rodrigo, S. Phenolic profiles and antioxidant properties of Physalis angulata L. as quality indicators. J. Pharm. Pharm. Res. 2017, 5, 114–128. [Google Scholar]
- Du, G.; Zhu, Y.; Wang, X.; Zhang, J.; Tian, C.; Liu, L.; Meng, Y.; Guo, Y. Phenolic composition of apple products and by-products based on cold pressing technology. J. Food Sci. Technol. 2019, 56, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, X.F. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, A.; Krisa, S.; Cluzet, S.; Da Costa, G.; Temsamani, H.; Renouf, E.; Mérillon, J.; Madani, K.; Mesnil, M.; Monvoisin, A.; et al. Phenolic contents and bioactive potential of peach fruit extracts. Food Chem. 2016, 202, 212–220. [Google Scholar] [CrossRef]
- Mendes, L.; de Freitas, V.; Baptista, P.; Carvalho, M. Comparative antihemolytic and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and fruit. Food Chem. Toxicol. 2011, 49, 2285–2291. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.; Saura-Calixto, F. A Procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Fiorentino, A.; Castaldi, S.; D’Abrosca, B.; Natale, A.; Carfora, A.; Messere, A.; Monaco, P. Polyphenols from the hydroalcoholic extract of Arbutus unedo living in a monospecific Mediterranean woodland. Biochem. Syst. Ecol. 2007, 35, 809–811. [Google Scholar] [CrossRef]
- Tavares, L.; Fortalezas, S.; Carrilho, C.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Santos, C.N. Antioxidant and antiproliferative properties of strawberry tree tissues. J. Berry Res. 2010, 1, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Jardim, C.; Macedo, D.; Figueira, I.; Dobson, G.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Menezes, R.; Santos, C.N. (Poly)phenol metabolites from Arbutus unedo leaves protect yeast from oxidative injury by activation of antioxidant and protein clearance pathways. J. Funct. Foods 2017, 32, 333–346. [Google Scholar] [CrossRef]
- Dib, M.A.; Allali, H.; Tabti, B.; Bendiabdellah, A.; Djabou, N. A new proanthocyanidins from Arbutus unedo L. stems. Asian J. Chem. 2008, 20, 3926–3934. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Jurica, K.; Brčić Karačonji, I.; Mikolić, A.; Milojković-Opsenica, D.; Benković, V.; Kopjar, N. In vitro safety assessment of the strawberry tree (Arbutus unedo L.) water leaf extract and arbutin in human peripheral blood lymphocytes. Cytotechnology 2018, 70, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Morgado, S.; Morgado, M.; Plácido, A.I.; Roque, F.; Duarte, A.P. Arbutus unedo L.: From traditional medicine to potential uses in modern pharmacotherapy. J. Ethnopharmacol. 2018, 225, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Dib, M.A.; Paolini, J.; Bendahou, M.; Varesi, L. Chemical composition of fatty acid and unsaponifiable fractions of leaves, stems and roots of arbutus unedo and in vitro antimicrobial activity of unsaponifiable extracts. Nat. Prod. Commun. 2010, 5, 2010. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Suárez, B.; Álvarez, Á.L.; García, Y.D.; del Barrio, G.; Lobo, A.P.; Parra, F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010, 120, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Łata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hortic. Amst. 2009, 121, 176–181. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Tuszyński, T. Antioxidant activity of apples-an impact of maturity stage and fruit part. Acta Sci. Pol. Technol. Aliment. 2011, 10, 443–454. [Google Scholar] [PubMed]
- Çam, M.; Aaby, K. Optimization of extraction of apple pomace phenolics with water by response surface methodology. J. Agric. Food Chem. 2010, 58, 9103–9111. [Google Scholar] [CrossRef]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Giménez-Bastida, J.A. Role of apple phytochemicals, phloretin and phloridzin, in modulating processes related to intestinal inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupasinghe, H.P.V.; Yasmin, A. Inhibition of oxidation of aqueous emulsions of Omega-3 fatty acids and fish oil by phloretin and phloridzin. Molecules 2010, 15, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Qiu, L.; Chen, X.; Song, K.-K.; Shi, Y.; Chen, Q.-X. Inhibitory effects of phloridzin dihydrate on the activity of mushroom (Agaricus bisporus) tyrosinase. Bioorg. Med. Chem. 2007, 15, 1568–1571. [Google Scholar] [CrossRef]
- Chang, T. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Bachra, K.; Nabil, K.; Karim, T.; Adjebli, A.; Dahmoune, F.; Maiza-Benabdeslam, F. Phenolic compounds from Citrus leaves: Antioxidant activity and enzymatic browning inhibition. J. Complement. Integr. Med. 2017, 14, 0030. [Google Scholar]
- Mcdougall, G.J.; Stewart, D. The inhibitory effects of berry polyphenols on digestive enzymes. Biofactors 2008, 23, 189–195. [Google Scholar] [CrossRef]
- Adera, K.T.; Inami, Y.M.; Akamatsu, K.T.; Atsuoka, T.M. Inhibition of α-Glucosidase and α-Amylase by Flavonoids. J Nutr Sci Vitam. 2006, 149–153. [Google Scholar]
- Le Bourvellec, C.; Le Quéré, J.; Sanoner, P.; Drilleau, J.F.; Guyot, S. Inhibition of apple polyphenol oxidase activity by procyanidins and polyphenol oxidation products. J. Agric. Food Chem. 2004, 52, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Rohn, S.; Rawel, H.M.; Kroll, J. Inhibitory effects of plant phenols on the activity of selected enzymes. J. Agric. Food Chem. 2002, 50, 3566–3571. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, T.; Meguro, S.; Kawachi, S. Purification and characterization of polyphenol oxidase from Henry chestnuts (Castanea henryi). J. Wood Sci. 2004, 50, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Pati, S.; Losito, I.; Palmisano, F.; Zambonin, P. Characterization of caffeic acid enzymatic oxidation by-products by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2006, 1102, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Soliva-Fortuny, R.C.; Biosca-Biosca, M.; Grigelmo-Miguel, N.; Martín-Belloso, O. Browning, polyphenol oxidase activity and headspace gas composition during storage of minimally processed pears using modified atmosphere packaging. J. Sci. Food Agric. 2002, 82, 1490–1496. [Google Scholar] [CrossRef]
- Pena, L.; Jiang, Y. Effects of chitosan coating on shelf life and quality of fresh-cut Chinese water chestnut. Lwt Food Sci. Technol. 2003, 36, 359–364. [Google Scholar] [CrossRef]
- Li-Qin, Z.; Zhou, J.; Zhu, S.-H.; Guo, L.-H. Inhibition of browning on the surface of peach slices by short-term exposure to nitric oxide and ascorbic acid. Food Chem. 2009, 114, 174–179. [Google Scholar] [CrossRef]
- Rigal, D.; Gauillard, F.; Richard-Forget, F. Changes in the carotenoid content of apricot (Prunus armeniaca, var Bergeron) during enzymatic browning: β-carotene inhibition of chlorogenic acid degradation. J. Sci. Agric. 2000, 80, 763–768. [Google Scholar] [CrossRef]
- Brecht, J.K. Physiology of Lightly Processed Fruits and Vegetables. HortScience 1995, 30, 18–22. [Google Scholar] [CrossRef]
- Taylor, S.L.; Higley, N.A.; Bush, R.K. Sulfites in foods: Uses, analytical methods, residues, fate, exposure assessment, metabolism, toxicity, and hypersensitivity. Adv. Food Res. 1986, 30, 1–76. [Google Scholar]
- Sapers, G.; Hicks, K.; Phillips, J.; Garzarella, L.; Pondish, D.; Matulaitis, R.; McCormack, T.; Sondey, S.; Seib, P.; Ei-Atawy, Y. Control of enzymatic browning in apple with ascorbic acid derivatives, polyphenol oxidase inhibitors, and complexing agents. J. Food Sci. 1989, 54, 997–1002. [Google Scholar] [CrossRef]
- Luo, Y.; Lu, S.; Zhou, B.; Feng, H. LWT—Food Science and Technology Dual effectiveness of sodium chlorite for enzymatic browning inhibition and microbial inactivation on fresh-cut apples. Lwt Food Sci. Technol. 2011, 44, 1621–1625. [Google Scholar] [CrossRef]
- Perez-gago, M.; Serra, M.; Río, M. Color change of fresh-cut apples coated with whey protein concentrate-based edible coatings. Postharvest Biol. Technol. 2006, 39, 84–92. [Google Scholar] [CrossRef]
- Fry, S.C.; Fry, S.C. Primary cell wall metabolism: Tracking the careers of wall polymers in living plant cells. New Phytologist. 2004, 161, 641–675. [Google Scholar] [CrossRef]
- Gomes, M.H.; Vieira, T.; Fundo, J.F.; Almeida, D.P.F. Polyphenoloxidase activity and browning in fresh-cut “Rocha” pear as affected by pH, phenolic substrates, and antibrowning additives. Postharvest Biol. Technol. 2014, 91, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.; Xuetog, F. Combination of sodium chlorite and calcium propionate reduces enzymatic browning and microbial population of fresh-cut “ Granny Smith ” apples. Food Microbiol. Saf. 2010, 75, 72–77. [Google Scholar] [CrossRef] [PubMed]



| Plant Name and Part | |
|---|---|
| Elderberry flower (Sambucus L.) | Vine (Vitis vinifera L.) leaves |
| Pear (Pyrus communis L. “Rocha”) pulp | Vine (Vitis vinifera L.) branches |
| Pear (Pyrus communis L. “Rocha”) peel | Olive (Oleo europaea L.) leaves |
| Pear (Pyrus communis L. “Rocha”) pomace | Olive (Oleo europaea L.) branches |
| Apple (Malus domestica L.) peel | Acorn (Quercus L.) bark |
| Apple (Malus domestica L.) pomace | Bitter Melon (Momordica charantia L.) whole plant |
| Strawberry tree (Arbutus Unedo L.) leaves | Potato plant (S.tuberosum L.) leaves |
| Strawberry tree (Arbutus Unedo L.) branches | |
| NES | Total Phenolic Content | Antioxidant Activity (AA) |
|---|---|---|
| TPC | IC50 | |
| Strawberry tree leaves | 207.97 ± 0.007 b | 0.65 ± 0.11 b |
| Strawberry tree branches | 104.07 ± 16.38 c | 0.75 ± 0.06 c |
| Apple byproduct | 6.76 ± 0.11 d | 45.59 ± 1.34 d |
| Positive control | ||
| Ascorbic acid | 938.35 ± 18.05 a | 0.25 ± 0.08 a |
| Postulate Compounds | Rt | [M-H]− | MS2 Ions (m/z) | Molecular | Strawberry Tree | Strawberry Tree | Apple |
|---|---|---|---|---|---|---|---|
| (min) | (m/z) | Formula | Leaves | Branches | Byproduct | ||
| Hydroxybenzoic acids | |||||||
| 1-O-Vanilloyl-β-D-glucose | 8.8 | 329.09 | 167 | C14H17O9 | √ | √ | √ |
| Vanillyl alcohol | 6.7 | 153.06 | 123 | C8H9O3 | nd | nd | √ |
| Gentisic acid | 6.5 | 153.02 | 109 | C7H5O4 | nd | √ | nd |
| Protocatechiuc acid | 6.2 | 315.07 | 108, 152 | C13H15O9 | nd | √ | nd |
| Protocatechualdehyde | 5.6 | 137.02 | 93 | C7H5O3 | nd | nd | √ |
| Gentisic acid 5-O-β-glucoside | 6.2 | 315.06 | 108, 151 | C13H15O9 | √ | nd | √ |
| Gentisic acid 2-O-β-glucoside | 5.6 | 315.07 | 109, 153 | C13H15O9 | nd | nd | √ |
| Gentisic acid derivative | 6.5 | 315.11 | 153 | C13H19O8 | √ | nd | nd |
| 4-Hydroxybenzoic acid | 5.6 | 137.02 | 93 | C7H5O3 | √ | nd | nd |
| 4-Hydroxybenzoate-β-D-glucoside | 5.4 | 299.08 | 9, 137 | C13H15O8 | √ | nd | nd |
| 4-Hydroxybenzoate-β-D-glucoside | 6.4 | 299.08 | 59, 137, 179 | C13H15O8 | √ | nd | nd |
| 1-O-Galloyl- β-D-glucose | 8.9 | 577.13 | 289, 407, 425 | C13H15O10 | √ | √ | nd |
| Gallic acid | 13.1 | 433.08 | 300 | C7H5O5 | √ | √ | nd |
| Digallic acid | 8.5 | 321.03 | 169 | C14H9O9 | nd | √ | nd |
| 4-O-Galloylshikimic acid | 12.3 | 939.11 | 769 | C14H13O9 | √ | √ | nd |
| Syringic acid | 7.6 | 577.14 | 289, 407 | C9H15O7 | √ | √ | nd |
| Hydroxycinnamic acids | |||||||
| p-Coumaric acid | 8.5 | 163.04 | 119 | C9H8O3 | √ | √ | √ |
| p-Coumaroyl-β-D-glucose | 8.5 | 325.10 | 119, 163 | C15H17O8 | √ | √ | √ |
| Caffeoylquinic acid | 8.4 | 353.12 | 191.05 | C16H18O9 | nd | nd | √ |
| 1,3 dicaffeoylquinic acid | 13.2 | 515.12 | 191 | C25H23O12 | nd | √ | nd |
| Caffeic acid 3-β−D-glucoside | 8.2 | 341.09 | 135, 179 | C15H17O9 | nd | nd | √ |
| 3,4-Caffeoyl alcohol-O-hexoside | 7.9 | 327.11 | 165 | C15H19O8 | nd | nd | √ |
| 1-O-Feruloyl-β-D-glucose | 9.2 | 355.10 | 175, 193 | C16H19O9 | nd | nd | √ |
| 1-O-Feruloyl-β-D-glucose | 10.2 | 355.10 | 175, 193, 235 | C16H19O9 | nd | nd | √ |
| Flavones | |||||||
| Isoorientin 2″-O-rhamnoside | 12.8 | 593.1512 | 285 | C27H29O15 | √ | √ | nd |
| 2″-O-Galloylorientin | 12.9 | 599.1042 | 169, 313, 447 | C28H23O15 | √ | nd | nd |
| Isoorientin 6″-O-p-hydroxybenzoate | 15.8 | 567.1144 | 284 | C28H23O13 | √ | nd | nd |
| Flavanols | |||||||
| Procyanidin dimer B type | 8.9 | 577.13 | 289, 407, 425 | C30H26O12 | √ | √ | √ |
| Ellagic acid 4-α-L-arabinofuranoside | 13.1 | 433.08 | 300 | C19H14O12 | nd | nd | √ |
| Penta-O-galloyl-β−D-glucose | 12.3 | 939.11 | 769 | C41H31O26 | √ | √ | nd |
| Procyanidin B5 | 7.6 | 577.14 | 289, 407 | C30H25O12 | √ | √ | nd |
| Procyanidin B4 | 7.9 | 577.14 | 125, 289, 407 | C30H25O122 | √ | √ | nd |
| Procyanidin B-5,3′-O-gallate | 9.7 | 729.15 | 289, 407, 577 | C34H29O16 | √ | √ | nd |
| Procyanidin B-13′-O-gallate | 10.3 | 729.15 | 289, 407, 577 | C37H29O16 | √ | √ | nd |
| (-)-Epicatechin gallate | 11.8 | 441.08 | 169, 289 | C22H17O10 | √ | √ | nd |
| (+)-Catechin | 8.3 | 289.07 | 109, 123 | C15H13O6 | √ | √ | nd |
| Flavonols | 13.4 | 447.09 | 300, 301 | ||||
| Kaempferol 3-O-β−D-glucoside | 12 | 463.09 | 300 | C21H19O11 | √ | √ | √ |
| Quercetin 3-O-β−D-glucoside | 12.2 | 463.09 | 300 | C21H19O12 | √ | √ | √ |
| Quercetin 3-O-β−D-galactoside | 12.8 | 593.15 | 285 | C21H19O12 | √ | √ | √ |
| Kaempferol-3-O-β−D-rutinoside | 13.4 | 447.09 | 300, 301 | C27H29O15 | nd | nd | √ |
| Kaempferol 3-O-β−D-xyloside | 14.4 | 417.08 | 284 | C20H17O10 | √ | √ | nd |
| Kaempferol 3-O-α L-rhamnoside | 14.8 | 431.10 | 285 | C21H20O10 | √ | nd | nd |
| Luteolin 7-O-β−D-glucoside | 13.4 | 447.09 | 300, 301 | C21H19O11 | √ | nd | nd |
| Quercetin derivative | 433.08 | C20H17O11 | √ | √ | nd | ||
| Quercetin hexose galloyl derivative | 11.3 | 615.10 | 300, 463 | C28H23O16 | √ | √ | nd |
| Myricetin 3-O-α−D-rhamnoside | 11.8 | 463.09 | 316 | C21H19O12 | √ | √ | nd |
| Rutin | 11.9 | 609.15 | 300, 301 | C27H29O16 | √ | √ | nd |
| 8-Hydroxykaempferol | 16.7 | 301.04 | 121, 151, 178 | C15H9O7 | √ | √ | nd |
| Quercitrin-2″-gallate | 13.1 | 599.10 | 169, 313, 463 | C28H23O16 | nd | √ | nd |
| Other Flavonoids | |||||||
| Taxifolin | 8.6 | 303.05 | 125, 153 | C15H11O7 | √ | √ | nd |
| Taxifolin | 13.6 | 303.05 | 151 | C15H11O7 | √ | √ | nd |
| Chalcone | |||||||
| Arbutin | 2.1 | 271.08 | 108 | C12H15O7 | √ | √ | nd |
| Dihydrochalcones | |||||||
| Phloretin glucoside | 13.2 | 567.17 | 273, 167 | C26H31O14 | nd | nd | √ |
| Phloridzin | 14.5 | 435.13 | 273 | C21H23O10 | nd | nd | √ |
| Postulate Compounds | Strawberry Tree | Strawberry Tree | Apple |
|---|---|---|---|
| Leaves | Branches | Byproduct | |
| Hydroxybenzoic acids | |||
| Protocatechiuc acid | nd | 1.15 ± 0.01 | 0.05 ± 0.01 |
| Flavanols | |||
| Procyanidin dimer B type | 8.78 ± 0.13 | 6.01 ± 1.10 | nd |
| Procyanidin dimer B type | nd | 5.53 ± 1.31 | nd |
| (-)-Epicatechin gallate | 2.76 ± 0.09 | 1.79 ± 0.07 | nd |
| (+)-Catechin | 10.18 ± 0.25 | 12.38 ± 1.21 | nd |
| Flavonols | |||
| Kaempferol 3-O-β−D-glucoside | 0.53 ± 0.01 | 2.17 ± 0.02 | 0.10 ± 0.001 |
| Kaempferol derivate | 9.90 ± 0.02 | nd | nd |
| Kaempferol derivate | 0.58 ± 0.004 | nd | nd |
| Quercetin 3-O-β−D-glucoside | 0.51 ± 0.003 | 0.55 ± 0.03 | 0.06 ± 0.001 |
| Quercetin 3-O-β−D-galactoside | 0.53 ± 0.001 | 0.29 ± 0.01 | 0.23 ± 0.002 |
| Luteolin 7-O-β−D-glucoside | 7.37 ± 0.01 | nd | nd |
| Quercetin derivative | 0.66 ± 0.003 | nd | nd |
| Quercetin derivative | 0.45 ± 0.001 | 0.52 ± 0.03 | nd |
| Rutin | 0.82 ± 0.01 | 0.42 ± 0.03 | nd |
| Phenolic acids | |||
| Gallic acid | 3.52 ± 0.32 | 4.85 ± 0.17 | nd |
| Syringic acid | 0.65 ± 0.36 | 1.24 ± 0.07 | nd |
| Gallic acid derivate | 1.08 ± 0.004 | 3.58 ± 0.24 | nd |
| Gallic acid derivate | 0.67 ± 0.05 | 0.57 ± 0.08 | nd |
| Gallic acid derivate | nd | 0.45 ± 0.01 | nd |
| Chalcone | |||
| Arbutin | 52.33 ± 0.61 | 7.43 ± 1.14 | nd |
| Dihydrochalcones | |||
| Phloridzin | nd | nd | 0.51± 0.01 |
| IC50 | Ascorbic Acid | Strawberry Tree Leaves | Strawberry Tree Branches | Apple Byproduct |
|---|---|---|---|---|
| PPO | 0.11 ± 0.02 a R2 = 0.998 | 53.92 ± 2.38 b R2 = 0.950 | 5.97 ± 0.73 b R2 = 0.927 | 127.30 ± 0.89 c R2 = 0.977 |
| POX | 0.52 ± 0.05 a R2 = 0.986 | 0.77 ± 0.10 a R2 = 0.999 | 2.25 ± 0.58 b R2 = 0.997 | 13.86 ± 0.76 c R2 = 0.947 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, C.; Fonseca, A.M.A.; Amaro, A.L.; Vilas-Boas, A.A.; Oliveira, A.; Santos, S.A.O.; Silvestre, A.J.D.; Rocha, S.M.; Isidoro, N.; Pintado, M. Natural-Based Antioxidant Extracts as Potential Mitigators of Fruit Browning. Antioxidants 2020, 9, 715. https://doi.org/10.3390/antiox9080715
Dias C, Fonseca AMA, Amaro AL, Vilas-Boas AA, Oliveira A, Santos SAO, Silvestre AJD, Rocha SM, Isidoro N, Pintado M. Natural-Based Antioxidant Extracts as Potential Mitigators of Fruit Browning. Antioxidants. 2020; 9(8):715. https://doi.org/10.3390/antiox9080715
Chicago/Turabian StyleDias, Cindy, Alexandre M. A. Fonseca, Ana L. Amaro, Ana A. Vilas-Boas, Ana Oliveira, Sonia A. O. Santos, Armando J. D. Silvestre, Sílvia M. Rocha, Nélson Isidoro, and Manuela Pintado. 2020. "Natural-Based Antioxidant Extracts as Potential Mitigators of Fruit Browning" Antioxidants 9, no. 8: 715. https://doi.org/10.3390/antiox9080715