Synthesis, In Vitro Antioxidant Properties and Distribution of a New Cyanothiophene-Based Phenolic Compound in Olive Oil-In-Water Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of N-(3-cyano-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-3,5-dihydroxybenzamide (SIM-53B)
2.3. Emulsion Preparation
2.4. Antioxidant Activity Determined by DPPH•, ABTS and CUPRAC Assays
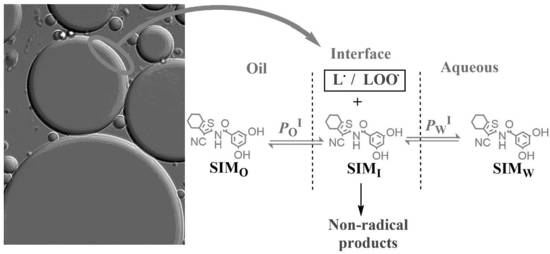
2.5. Pseudophase Kinetic Model Applied to Emulsions: Partition Constants and Distribution of SIM-53B
2.6. Determination of kobs in Opaque Emulsion by Spectrophotometry
2.7. Statistical Analysis
3. Results and Discussion
3.1. Partition Constants Values PWI and POI for SIM-53B
3.2. Distribution of SIM-53B and Its Local Effective Concentrations: Effect of Emulsifier Volume Fraction and O/W Ratio
3.3. Radical Scavenging Activity of SIM-53B
3.4. Prediction of Dug-Like Properties Pharmacokinetic Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Webb, T.R. Improving the Efficiency of the Drug Development by Expanding the Scope of the Role of Medicinal Chemists in Drug Discovery. ACS Med. Chem. Lett. 2018, 9, 1153–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrast, M.; Anderluh, M.; Knez, D.; Randall, C.P.; Barreteau, H.; O’Neill, A.J.; Blanot, D.; Gobec, S. Design, synthesis and evaluation of second generation MurF inhibitors based on a cyanothiophene scaffold. Eur. J. Med. Chem. 2014, 73, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Rauter, A.P. Membrane-targeting antibiotics: Recent developments outside the peptide space. Future Med. Chem. 2019, 11, 254–280. [Google Scholar] [CrossRef]
- Malin, J.J.; de Leeuw, E. Therapeutic compounds targeting Lipid II for antibacterial purposes. Infect. Drug Resist. 2019, 12, 2613–2625. [Google Scholar] [CrossRef] [Green Version]
- Mandal, M.; Wu, Y.; Misiaszek, J.; Li, G.; Buevich, A.; Caldwell, J.P.; Liu, X.; Mazzola, R.D.; Orth, P.; Strickland, C.; et al. Structure-Based Design of an Iminoheterocyclic β-Site Amyloid Precursor Protein Cleaving Enzyme (BACE) Inhibitor that Lowers Central Aβ in Nonhuman Primates. J. Med. Chem. 2016, 59, 3231–3248. [Google Scholar] [CrossRef]
- Hrast, M.; Turk, S.; Sosic, I.; Knez, D.; Randall, C.P.; Barreteau, H.; Contreras-Martel, C.; Dessen, A.; O’Neill, A.J.; Mengin-Lecreulx, D.; et al. Structure activity relationships of new cyanothiophene inhibitors of the essential peptidoglycan biosynthesis enzyme MurF. Eur. J. Med. Chem. 2013, 66, 32–45. [Google Scholar] [CrossRef]
- El-Meligie, S.E.M.; Khalil, N.A.; El-Nassan, H.B.; Ibraheem, A.A.M. Efficient synthesis of new 3-amino-4-cyanothiophene derivatives. Chem. Pap. 2020, 74, 2491–2500. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Aldawsari, F.D.; Al-Showiman, S.S.; Barakat, A.; Soliman, S.M.; Choudhary, M.I.; Yousuf, S.; Hadda, T.B.; Mubarak, M.S. Synthesis, Molecular Structure Optimization, and Cytotoxicity Assay of a Novel 2-Acetyl-3-amino-5-[(2-oxopropyl)sulfanyl]-4-cyanothiophene. Molecules 2016, 21, 214. [Google Scholar] [CrossRef] [Green Version]
- Azam, M.A.; Jupudi, S. Insight into the structural requirements of thiophene-3-carbonitriles-based MurF inhibitors by 3D-QSAR, molecular docking and molecular dynamics study. J. Recept Signal Transduct. Res. 2017, 37, 522–534. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Artiga-Artigas, M.; Molet-Rodríguez, A.; Turmo-Ibarz, A.; Martín-Belloso, O. Emulsion-Based Nanostructures for the Delivery of Active Ingredients in Foods. Front. Sustain. Food Syst. 2018, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J. Enhanced delivery of lipophilic bioactives using emulsions: A review of major factors affecting vitamin, nutraceutical and lipid bioaccessibility. Food Funct. 2018, 9, 22–41. [Google Scholar] [CrossRef]
- Lodhi, S. Nanoemulsion: A brief review on development and application in Parenteral Drug Delivery. Adv. Pharm. J. 2018, 3, 43–54. [Google Scholar]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef] [Green Version]
- Chime, S.A.; Knechukwu, F.C.; Attama, A.A. Nanoemulsions—Advances in formulation, characterization and applications in drug delivery. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; Intech: Istambul, Turkey, 2014; Volume 3, pp. 77–126. [Google Scholar]
- Srinivas Ganta, S.; Singh, A.; Patel, N.R.; Cacaccio, P.; Rawal, Y.H.; Davis, B.J.; Amiji, M.M.; Coleman, T.P. Development of EGFR-Targeted Nanoemulsion for Imaging and Novel Platinum Therapy of Ovarian Cancer. Pharm. Res. 2014, 31, 2490–2502. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Park, Y.J. Paclitaxel-loaded hyaluronan solid nanoemulsions for enhanced treatment efficacy in ovarian cancer. Int. J. Nanomed. 2017, 12, 645–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattos, C.; Argenta, D.; Melchiades, G.; Sechini Cordeiro, M.N.; Tonini, M.; Hoehr Moraes, M.; Weber, T.B.; Souza Roman, S.; Nunes, R.J.; Ferreira Teixeira, H.; et al. Nanoemulsions containing a synthetic chalcone as an alternative for treating cutaneous leshmaniasis: Optimization using a full factorial design. Int. J. Nanomed. 2015, 10, 5529–5542. [Google Scholar]
- Moura, J.A.; Valduga, C.J.; Tavares, E.R.; Kretzer, L.F.; Maria, A.D.; Maranhão, R.C. Novel formulation of a methotrexate derivative with a lipid nanoemulsion. Int. J. Nanomed. 2011, 6, 2285–2295. [Google Scholar]
- Tan, S.L.; Stanslas, J.; Basri, M.; Karjiban, R.A.A.; Kirby, B.P.; Sani, D.; Basri, H.B. Nanoemulsion-based Parenteral Drug Delivery System of Carbamazepine: Preparation, Characterization, Stability Evaluation and Blood-Brain Pharmacokinetics. Curr. Drug Deliv. 2015, 12, 795–804. [Google Scholar] [CrossRef]
- Brussel, F.; Manoela, L.; Luisa, B.W.; Michelle, F.; Koester, L.S.; Teixeira, H.F. Nanoemulsions as parenteral drug delivery systems: A review. Chem. New 2012, 35, 34–39. [Google Scholar]
- Bravo-Díaz, C.; Romsted, L.S.; Liu, C.; Losada-Barreiro, S.; Pastoriza-Gallego, M.J.; Gao, X.; Gu, Q.; Krishnan, G.; Sánchez-Paz, V.; Zhang, Y.; et al. To Model Chemical Reactivity in Heterogeneous Emulsions, Think Homogeneous Microemulsions. Langmuir 2015, 31, 8961–8979. [Google Scholar]
- Berton-Carabin, C.C.; Ropers, M.H.; Genot, C. Lipid Oxidation in Oil-in-Water Emulsions: Involvement of the Interfacial Layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo-Díaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Costa, M.; Losada-Barreiro, S.; Paiva Martins, F.; Bravo-Díaz, C. Modulating the interfacial concentration of gallates to improve the oxidative stability of fish oil-in-water emulsions. Food Res. Int. 2018, 112, 192–198. [Google Scholar] [CrossRef]
- Meireles, M.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Monteiro, L.S. Control of antioxidant efficiency of chlorogenates in emulsions: Modulation of antioxidant interfacial concentrations. J. Sci. Food Agric. 2019, 99, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Physical evidence that the variations in the efficiency of homologous series of antioxidants in emulsions are due to differences in their partitioning. J. Sci. Food Agric. 2017, 97, 564–571. [Google Scholar] [CrossRef]
- Silva, R.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Partitioning and antioxidative effect of protocatechuates in soybean oil emulsions: Relevance of emulsifier concentration. Eur. J. Lipid Sci. Technol. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Almeida, J.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. Interfacial Concentrations of Hydroxytyrosol and Its Lipophilic Esters in Intact Olive Oil-in-Water Emulsions: Effects of Antioxidant Hydrophobicity, Surfactant Concentration, and the Oil-to-Water Ratio on the Oxidative Stability of the Emulsions. J. Agric. Food Chem. 2016, 64, 5274–5283. [Google Scholar] [CrossRef]
- Lesser, R. Über die Acetylierung aromatischer Oxy-carbonsäuren. Ber. Dtsch. Chem. Ges. B. 1926, 59B, 233–236. [Google Scholar] [CrossRef]
- Knez, D.; Coquelle, N.; Pišlar, A.; Žakelj, S.; Jukič, M.; Sova, M.; Mravljak, J.; Nachon, F.; Brazzolotto, X.; Kos, J.; et al. Multi-target-directed ligands for treating Alzheimer’s disease: Butyrylcholinesterase inhibitors displaying antioxidant and neuroprotective activities. Eur. J. Med. Chem. 2018, 156, 598–617. [Google Scholar] [CrossRef]
- Thaiponga, K.; Boonprakoba, U.; Crosbyb, K.; Cisneros-Zevallosc, L.; Hawkins Byrnec, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Apak, R.; Guçlu, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Paz, V.; Pastoriza-Gallego, M.J.; Losada-Barreiro, S.; Bravo-Diaz, C.; Gunaseelan, K.; Romsted, L.S. Quantitative determination of a-tocopherol distribution in a tributyrin/Brij 30/water model food emulsion. J. Colloid. Interface Sci. 2008, 320, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.swissadme (accessed on 10 June 2020).
- Losada-Barreiro, S.; Bravo Díaz, C.; Paiva Martins, F.; Romsted, L.S. Maxima in antioxidant distributions and efficiencies with increasing hydrophobicity of gallic acid and its alkyl esters. The pseudophase model interpretation of the “Cut-off effect”. J. Agric. Food Chem. 2013, 61, 6533–6543. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; Garcia-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and Cinnamic Acid Derivatives as Antioxidants: Structure−Activity Relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Choi, G.H.; Ro, J.H.; Park, B.J.; Lee, D.Y.; Cheong, M.S.; Lee, D.Y.; Seo, W.D.; Kim, J.H. Benzaldehyde as a new class plant growth regulator on Brassica campestris. J. Appl. Biol. Chem. 2016, 59, 159–164. [Google Scholar] [CrossRef]
- Sendra, J.M.; Sentandreu, E.; Navarro, J.L. Reduction kinetics of the free stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH•) for determination of the antiradical activity of citrus juices. Eur. Food Res. Technol. 2006, 223, 615–621. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, druglikeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2018, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Krüger, A.; Gonçalves Maltarollo, V.; Wrenger, C.; Kronenberger, T. ADME Profiling in Drug Discovery and a New Path Paved on Silica. In Drug Discovery and Development—New Advances; Gaitonde, V., Karmakar, R., Trivedi, A., Eds.; IntechOpen: London, UK, 2019; Volume 1, pp. 1–32. [Google Scholar]






| PWI | 3227 ± 426 |
| POI | 375 ± 49 |
| kI (M−1s−1) | 0.0252 ± 0.0077 |
| EC50(DPPH) (μM) | 440.8 ± 48.9 |
| EC50(ABTS) (μM) | 6.45 ± 0.60 |
| TEACcuprac | 1.04 (rt); 1.81 (50 °C) |
| Log PWOCT * | 2.73 |
| Solubility * | Moderately soluble |
| Drug likeness * | Yes |
| Bioavailability score * | 0.55 |
| GI Absorption * | High |
| BBB permeation * | No |
| Pg-p substrate * | No |
| CYP1A2 Inhibitor * | Yes |
| CYP2C19 Inhibitor * | Yes |
| CYP2C9 Inhibitor * | Yes |
| CYP2D6 Inhibitor * | No |
| CYP3A4 Inhibitor * | Yes |
| Log kp (skin permeation) * | −5.69 cm/s |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losada-Barreiro, S.; Sova, M.; Mravljak, J.; Saso, L.; Bravo-Díaz, C. Synthesis, In Vitro Antioxidant Properties and Distribution of a New Cyanothiophene-Based Phenolic Compound in Olive Oil-In-Water Emulsions. Antioxidants 2020, 9, 623. https://doi.org/10.3390/antiox9070623
Losada-Barreiro S, Sova M, Mravljak J, Saso L, Bravo-Díaz C. Synthesis, In Vitro Antioxidant Properties and Distribution of a New Cyanothiophene-Based Phenolic Compound in Olive Oil-In-Water Emulsions. Antioxidants. 2020; 9(7):623. https://doi.org/10.3390/antiox9070623
Chicago/Turabian StyleLosada-Barreiro, Sonia, Matej Sova, Janez Mravljak, Luciano Saso, and Carlos Bravo-Díaz. 2020. "Synthesis, In Vitro Antioxidant Properties and Distribution of a New Cyanothiophene-Based Phenolic Compound in Olive Oil-In-Water Emulsions" Antioxidants 9, no. 7: 623. https://doi.org/10.3390/antiox9070623