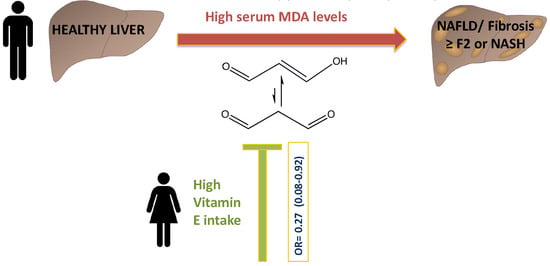
Serum Malondialdehyde is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Definition of Hepatic and Metabolic Variables
2.3. Nutritional and Lifestyle Variables Evaluation and Definitions
2.4. Determination of Malondialdehyde Serum Levels
2.5. Statistical Analysis
3. Results
3.1. Description of the Study Population and Comparison between Subjects with High (by Median) and Low MDA
3.2. Dose-Response Association of MDA Levels and NAFLD among the Entire Study Sample and by Gender
3.3. Multivariate Association of Serum MDA Levels and NAFLD and Presumed Related Liver Damage Stratified by Gender
3.4. Multivariate Association of Vitamins E and C Intake and Serum MDA Levels Stratified by Gender
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellentani, S.; Tiribelli, C. Is it time to change NAFLD and NASH nomenclature? Lancet Gastroenterol. Hepatol. 2017, 2, 547–548. [Google Scholar] [CrossRef]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef]
- Bellanti, F.; Villani, R.; Facciorusso, A.; Vendemiale, G.; Serviddio, G. Lipid oxidation products in the pathogenesis of non-alcoholic steatohepatitis. Free Radic. Biol. Med. 2017, 111, 173–185. [Google Scholar] [CrossRef]
- Hardwick, R.N.; Fisher, C.D.; Canet, M.J.; Lake, A.D.; Cherrington, N.J. Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab. Dispos. 2010, 38, 2293–2301. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ucar, F.; Sezer, S.; Erdogan, S.; Akyol, S.; Armutcu, F.; Akyol, O. The relationship between oxidative stress and nonalcoholic fatty liver disease: Its effects on the development of nonalcoholic steatohepatitis. Redox Rep. 2013, 18, 127–133. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Faghihimani, E.; Adibi, P. Nonalcoholic fatty liver disease: Diagnostic biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Yesilova, Z.; Yaman, H.; Oktenli, C.; Ozcan, A.; Uygun, A.; Cakir, E.; Sanisoglu, S.Y.; Erdil, A.; Ates, Y.; Aslan, M.; et al. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. Am. J. Gastroenterol. 2005, 100, 850–855. [Google Scholar] [CrossRef]
- Varma, M.; Makwane, H.; Kare, P.; Jha, R.; Parmar, A. Study of serum ferritin, serum uric acid and plasma malondialdehyde (MDA) levels in non-alcoholic fatty liver disease. Int. J. Biomed. Adv. Res. 2016, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Albano, E.; Mottaran, E.; Vidali, M.; Reale, E.; Saksena, S.; Occhino, G.; Burt, A.; Day, C. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut 2005, 54, 987–993. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Duseja, A.; Das, A.; Dhiman, R.K.; Chawla, Y.K.; Kohli, K.K.; Bhansali, A. Patients with nonalcoholic fatty liver disease (NAFLD) have higher oxidative stress in comparison to chronic viral hepatitis. J. Clin. Exp. Hepatol. 2013, 3, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Gee, P.T. Unleashing the untold and misunderstood observations on vitamin E. Genes Nutr. 2011, 6, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hadi, H.; Vettor, R.; Rossato, M. Vitamin E as a Treatment for Nonalcoholic Fatty Liver Disease: Reality or Myth? Antioxidants 2018, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Ni, Y.; Nagata, N.; Xu, L.; Ota, T. Micronutrient Antioxidants and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 1379. [Google Scholar] [CrossRef] [Green Version]
- Phung, N.; Pera, N.; Farrell, G.; Leclercq, I.; Hou, J.Y.; George, J. Pro-oxidant-mediated hepatic fibrosis and effects of antioxidant intervention in murine dietary steatohepatitis. Int. J. Mol. Med. 2009, 24, 171–180. [Google Scholar]
- Nan, Y.M.; Wu, W.J.; Fu, N.; Liang, B.L.; Wang, R.Q.; Li, L.X.; Zhao, S.X.; Zhao, J.M.; Yu, J. Antioxidants vitamin E and 1-aminobenzotriazole prevent experimental non-alcoholic steatohepatitis in mice. Scand. J. Gastroenterol. 2009, 44, 1121–1131. [Google Scholar] [CrossRef]
- Wei, J.; Lei, G.H.; Fu, L.; Zeng, C.; Yang, T.; Peng, S.F. Association between Dietary Vitamin C Intake and Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study among Middle-Aged and Older Adults. PLoS ONE 2016, 11, e0147985. [Google Scholar] [CrossRef]
- Han, J.M.; Jo, A.N.; Lee, S.M.; Bae, H.S.; Jun, D.W.; Cho, Y.K.; Suk, K.T.; Yoon, J.H.; Ahn, S.B.; Cho, Y.J.; et al. Associations between intakes of individual nutrients or whole food groups and non-alcoholic fatty liver disease among Korean adults. J. Gastroenterol. Hepatol. 2014, 29, 1265–1272. [Google Scholar] [CrossRef]
- Da Silva, H.E.; Arendt, B.M.; Noureldin, S.A.; Therapondos, G.; Guindi, M.; Allard, J.P. A cross-sectional study assessing dietary intake and physical activity in Canadian patients with nonalcoholic fatty liver disease vs healthy controls. J. Acad. Nutr. Diet. 2014, 114, 1181–1194. [Google Scholar] [CrossRef]
- Chan, R.; Wong, V.W.; Chu, W.C.; Wong, G.L.; Li, L.S.; Leung, J.; Chim, A.M.; Yeung, D.K.; Sea, M.M.; Woo, J.; et al. Diet-Quality Scores and Prevalence of Nonalcoholic Fatty Liver Disease: A Population Study Using Proton-Magnetic Resonance Spectroscopy. PLoS ONE 2015, 10, e0139310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilett, W. Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Keinan-Boker, L.; Noyman, N.; Chinich, A.; Green, M.S.; Nitzan-Kaluski, D. Overweight and obesity prevalence in Israel: Findings of the first national health and nutrition survey (MABAT). Isr. Med. Assoc. J.: IMAJ 2005, 7, 219–223. [Google Scholar] [PubMed]
- Gore, R. Diffuse liver disease. In Textbook of Gastrointestinal Radiology; Gore, R.M., Levin, M.S., Laufer, I., Eds.; Saunders: Philadelphia, PA, USA, 1994; pp. 1968–2017. [Google Scholar]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Goldsmith, R.; Webb, M.; Blendis, L.; Halpern, Z.; Oren, R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): A population based study. J. Hepatol. 2007, 47, 711–717. [Google Scholar] [CrossRef]
- Munteanu, M.; Tiniakos, D.; Anstee, Q.; Charlotte, F.; Marchesini, G.; Bugianesi, E.; Trauner, M.; Romero Gomez, M.; Oliveira, C.; Day, C.; et al. Diagnostic performance of FibroTest, SteatoTest and ActiTest in patients with NAFLD using the SAF score as histological reference. Aliment. Pharmacol. Ther. 2016, 44, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Munteanu, M.; Charlotte, F.; Perazzo, H.; Ngo, Y.; Deckmyn, O.; Pais, R.; Merrouche, W.; de Ledinghen, V.; Mathurin, P.; et al. Diagnostic performance of a new noninvasive test for nonalcoholic steatohepatitis using a simplified histological reference. Eur. J. Gastroenterol. Hepatol. 2018, 30, 569–577. [Google Scholar] [CrossRef]
- Poynard, T.; Munteanu, M.; Deckmyn, O.; Ngo, Y.; Drane, F.; Messous, D.; Castille, J.M.; Housset, C.; Ratziu, V.; Imbert-Bismut, F. Applicability and precautions of use of liver injury biomarker FibroTest. A reappraisal at 7 years of age. BMC Gastroenterol. 2011, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008, 22, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss Isakov, N.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef]
- Chalasani, N.; Deeg, M.A.; Crabb, D.W. Systemic Levels of Lipid Peroxidation and Its Metabolic and Dietary Correlates in Patients with Nonalcoholic Steatohepatitis; LWW: Philadelphia, PA, USA, 2004. [Google Scholar]
- Klisic, A.; Isakovic, A.; Kocic, G.; Kavaric, N.; Jovanovic, M.; Zvrko, E.; Skerovic, V.; Ninic, A. Relationship between Oxidative Stress, Inflammation and Dyslipidemia with Fatty Liver Index in Patients with Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2018, 126, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Vanjiappan, S.; Hamide, A.; Ananthakrishnan, R.; Periyasamy, S.G.; Mehalingam, V. Nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and its association with cardiovascular disease. Diabetes Metab. Syndr. 2018, 12, 479–482. [Google Scholar] [CrossRef]
- Koroglu, E.; Canbakan, B.; Atay, K.; Hatemi, I.; Tuncer, M.; Dobrucali, A.; Sonsuz, A.; Gultepe, I.; Senturk, H. Role of oxidative stress and insulin resistance in disease severity of non-alcoholic fatty liver disease. Turk. J. Gastroenterol. 2016, 27, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Swiderska, M.; Maciejczyk, M.; Zalewska, A.; Pogorzelska, J.; Flisiak, R.; Chabowski, A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic. Res. 2019, 53, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gomez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, L.N.; Temm, C.J.; Saxena, R.; Vuppalanchi, R.; Schauer, P.; Rabinovitz, M.; Krasinskas, A.; Chalasani, N.; Mattar, S.G. Bariatric surgery-induced weight loss reduces hepatic lipid peroxidation levels and affects hepatic cytochrome P-450 protein content. Ann. Surg. 2010, 251, 1041–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kani, A.H.; Alavian, S.M.; Esmaillzadeh, A.; Adibi, P.; Azadbakht, L. Effects of a novel therapeutic diet on liver enzymes and coagulating factors in patients with non-alcoholic fatty liver disease: A parallel randomized trial. Nutrition 2014, 30, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Razavi Zade, M.; Telkabadi, M.H.; Bahmani, F.; Salehi, B.; Farshbaf, S.; Asemi, Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: A randomized clinical trial. Liver Int. 2016, 36, 563–571. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.X.; Huang, X.; Li, Y.; Sun, T.; Zang, S.; Guan, K.L.; Xiong, Y.; Liu, J.; Yuan, H.X. Targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity. Liver Int. 2020. [Google Scholar] [CrossRef]
- Jiang, J.X.; Fish, S.R.; Tomilov, A.; Li, Y.; Fan, W.; Dehnad, A.; Gae, D.; Das, S.; Mozes, G.; Charville, G.W.; et al. Non-phagocytic Activation of NOX2 is Implicated in Progressive Non-alcoholic Steatohepatitis During Aging. Hepatology 2020. [Google Scholar] [CrossRef]
- Mladenović, J.; Ognjanović, B.; Đorđević, N.; Matić, M.; Knežević, V.; Štajn, A.; Saičić, Z. Protective effects of oestradiol against cadmium-induced changes in blood parameters and oxidative damage in rats. Arch. Ind. Hyg. Toxic. 2014, 65, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oztekin, E.; Tiftik, A.M.; Baltaci, A.K.; Mogulkoc, R. Lipid peroxidation in liver tissue of ovariectomized and pinealectomized rats: Effect of estradiol and progesterone supplementation. Cell Biochem. Funct. 2007, 25, 401–405. [Google Scholar] [CrossRef]
- Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Salomone, F.; Webb, M.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. Dietary vitamin E and C intake is inversely associated with the severity of nonalcoholic fatty liver disease. Dig. Liver Dis. 2019, 51, 1698–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef] [Green Version]
- National Academy of Sciences. Dietary Reference Intakes (DRIs): Recommended Dietary Allowances and Adequate Intakes, Vitamins. 2011. Available online: www.ncbi.nlm.nih.gov/books/NBK56068/table/summarytables.t2/?report=objectonly (accessed on 1 January 2020).
- Miller, E.R., 3rd; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schurks, M.; Glynn, R.J.; Rist, P.M.; Tzourio, C.; Kurth, T. Effects of vitamin E on stroke subtypes: Meta-analysis of randomised controlled trials. BMJ 2010, 341, c5702. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Perumpail, B.J.; Li, A.A.; John, N.; Sallam, S.; Shah, N.D.; Kwong, W.; Cholankeril, G.; Kim, D.; Ahmed, A. The Role of Vitamin E in the Treatment of NAFLD. Diseases 2018, 6, 86. [Google Scholar] [CrossRef] [Green Version]
- Verdelho Machado, M.; Diehl, A.M. Role of Hedgehog Signaling Pathway in NASH. Int. J. Mol. Sci. 2016, 17, 857. [Google Scholar] [CrossRef] [Green Version]



|
Variable (Units, Normal Range) | Women | Men | ||||
|---|---|---|---|---|---|---|
| MDA < 13.46 (n = 92) | MDA ≥ 13.46 (n = 93) | p Value | MDA < 12.10 (n = 104) | MDA ≥ 12.10 (n = 105) | p Value | |
| Age (years) | 58.50 ± 6.92 | 59.73 ± 6.26 | 0.206 | 59.06 ± 6.59 | 59.30 ± 6.09 | 0.787 |
| BMI (Kg/m2) | 28.76 ± 6.20 | 26.53 ± 4.43 | 0.006 | 29.45 ± 6.19 | 28.50 ± 4.65 | 0.214 |
| Waist Circumference (cm) | 96.27 ± 14.55 | 92.07 ± 11.95 | 0.033 | 105.27 ± 12.57 | 103.42 ± 13.50 | 0.309 |
| HOMA-IR (score) | 2.61 ± 1.86 | 2.49 ± 2.10 | 0.682 | 3.05 ± 2.14 | 3.04 ± 1.89 | 0.990 |
| HbA1C (%) | 5.82 ± 0.50 | 5.85 ± 0.61 | 0.671 | 5.77 ± 0.56 | 6.10 ± 1.01 | 0.004 |
| Triglyceride (mg/dl) | 101.73 ± 48.39 | 107.44 ± 53.15 | 0.446 | 122.70 ± 81.71 | 116.04 ± 76.63 | 0.544 |
| Total Cholesterol (mg/dl) | 186.13 ± 33.18 | 192.48 ± 38.62 | 0.232 | 167.73 ± 30.66 | 171.30 ± 36.14 | 0.443 |
| ALT (U/L, 5-39) | 22.70 ± 13.51 | 24.38 ± 10.08 | 0.338 | 23.92 ± 11.29 | 27.54 ± 11.65 | 0.024 |
| AST (U/L, 7-40) | 22.81 ± 11.16 | 26.19 ± 10.30 | 0.033 | 22.38 ± 6.15 | 25.10 ± 7.00 | 0.003 |
| GGT (U/L, 6-28) | 29.85 ± 37.65 | 25.25 ± 27.62 | 0.345 | 27.91 ± 18.18 | 29.50 ± 28.96 | 0.637 |
| NashTest (score) | 0.40 ± 0.17 | 0.45 ± 0.15 | 0.022 | 0.42 ± 0.12 | 0.48 ± 0.13 | 0.001 |
| NASH (%) | 28.10 | 31.50 | 0.623 | 23.20 | 40.20 | 0.010 |
| FibroTest (score) | 0.16 ± 0.12 | 0.14 ± 0.11 | 0.291 | 0.25 ± 0.16 | 0.27 ± 0.17 | 0.405 |
| Significant Fibrosis (%) | 2.20 | 2.20 | 1.00 | 7.10 | 12.70 | 0.179 |
| Uric Acid (mg/dl, 2.3-6) | 4.88 ± 1.20 | 4.87 ± 1.23 | 0.982 | 5.93 ± 1.23 | 6.07 ± 1.19 | 0.401 |
| C-Reactive Protein (mg/L, <5) | 4.15 ± 4.91 | 3.99 ± 5.43 | 0.837 | 4.10 ± 7.63 | 3.71 ± 6.02 | 0.676 |
| Nutritional and lifestyle habits | ||||||
| Energy (Kcal) | 2153.82 ± 702.57 | 1971. 84 ± 644.25 | 0.068 | 2253. 95 ± 740.16 | 2031. 67 ± 712.68 | 0.028 |
| Saturate Fatty Acid (% of total Kcal/d) | 12.48 ± 3.97 | 12.34 ± 4.68 | 0.827 | 11.49 ± 3.24 | 12.54 ± 3.93 | 0.036 |
| Cholesterol (mg/d) | 337.28 ± 225.02 | 290.57 ± 131.96 | 0.088 | 367.35 ± 195.32 | 364.40 ± 239.01 | 0.922 |
| Fiber (gr/d) | 23.95 ± 12.98 | 23.17 ± 10.86 | 0.659 | 23.95 ± 13.07 | 21.60 ± 12.45 | 0.184 |
| Red/Processed Meat (portions/d) | 0.40 ± 0.66 | 0.33 ± 0.44 | 0.428 | 0.90 ± 1.13 | 0.76 ± 1.01 | 0.340 |
| Total Fish (portions/d) | 0.42 ± 0.48 | 0.55 ± 0.68 | 0.151 | 0.51 ± 0.46 | 0.58 ± 0.54 | 0.332 |
| Sugared Beverages (cups/d) | 3.71 ± 4.09 | 2.11 ± 3.61 | 0.005 | 3.87 ± 3.96 | 2.02 ± 3.31 | < 0.001 |
| Coffee (cups/d) | 2.56 ± 2.82 | 3.49 ± 3.46 | 0.048 | 2.41 ± 2.71 | 3.48 ± 3.47 | 0.014 |
| Alcohol (portions/d) | 0.73 ± 1.79 | 0.76 ± 1.51 | 0.902 | 2.55 ± 3.63 | 2.42 ± 3.53 | 0.785 |
| Vitamin E (mg/ 1000 Kcal) | 9.65 ± 6.77 | 7.53 ± 6.64 | 0.033 | 9.28 ± 6.67 | 6.16 ± 5.44 | < 0.001 |
| Vitamin C (mg/ 1000 Kcal) | 89.41 ± 58.54 | 91.78 ± 71.94 | 0.806 | 92.20 ± 61.17 | 79.01 ± 46.51 | 0.081 |
| Pack Years * | 11.23 ± 18.66 | 12.19 ± 23.42 | 0.758 | 19.86 ± 25.24 | 18.68 ± 25.30 | 0.735 |
| Physical Activity (hours/week) | 1.60 ± 2.66 | 2.37 ± 3.21 | 0.076 | 1.90 ± 3.52 | 2.76 ± 3.54 | 0.079 |
| NAFLD | NASH | Fibrosis ≥ F2 or NASH | |
|---|---|---|---|
| OR (95% CI), P | |||
| All Samples (n Cases) a | 146 | 117 | 121 |
| Model A | |||
| <12.87 | 1 (ref) | 1 (ref) | 1 (ref) |
| ≥12.87 | 2.24 (1.38–3.64), 0.001 | 1.51 (0.93–2.44), 0.094 | 1.46 (0.91–2.36). 0.121 |
| Model B | |||
| <12.87 | 1 (ref) | 1 (ref) | 1 (ref) |
| ≥12.87 | 1.93 (1.15–3.24), 0.013 | 1.14 (0.69–1.90), 0.612 | 1.12 (0.67–1.86), 0.666 |
| Women (n Cases) | 62 | 53 | 53 |
| Model A | |||
| <13.46 | 1 (ref) | 1 (ref) | 1 (ref) |
| ≥13.46 | 1.69 (0.79–3.63), 0.174 | 1.10 (0.53–2.31), 0.794 | b |
| Model B | |||
| <13.46 | 1 (ref) | 1 (ref) | 1 (ref) |
| ≥13.46 | 1.37 (0.60–3.14), 0.461 | 0.84 (0.37–1.87), 0.661 | b |
| Men (n Cases) | 84 | 64 | 68 |
| Model A | |||
| <12.10 | 1 (ref) | 1 (ref) | 1 (ref) |
| ≥12.10 | 2.87 (1.53–5.37), 0.001 | 2.20 (1.16–4.21), 0.017 | 2.22 (1.17–4.21), 0.015 |
| Model B | |||
| <12.10 | 1 (ref) | 1 (ref) | 1 (ref) |
| ≥12.10 | 2.59 (1.33–5.07), 0.005 | 1.95 (0.98–3.91), 0.059 | 2.04 (1.02–4.06), 0.043 |
| Variable | All Sample a | Women | Men | |||
|---|---|---|---|---|---|---|
| Value | OR (95% CI) P | Value | OR (95% CI) P | Value | OR (95% CI) P | |
| Vitamin E (>upper tertile, mg/1000 Kcal) | <8.43 | 1 (ref) | <8.40 | 1 (ref) | <8.48 | 1 (ref) |
| ≥8.43 | 0.28 (0.13–0.62) 0.002 | ≥8.40 | 0.27 (0.08–0.92) 0.036 | ≥8.48 | 0.37 (0.13–1.08) 0.068 | |
| Vitamin C (>upper tertile, mg/1000 Kcal) intake | <97.83 | 1 (ref) | <102.84 | 1 (ref) | <94.93 | 1 (ref) |
| ≥97.83 | 1.12 (0.63–1.97) 0.707 | ≥102.84 | 0.88 (0.39–2.00) 0.766 | ≥94.93 | 1.03 (0.47–2.24) 0.947 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Hahn, M.; Webb, M.; Shibolet, O.; Kariv, R.; Tirosh, O. Serum Malondialdehyde is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women. Antioxidants 2020, 9, 578. https://doi.org/10.3390/antiox9070578
Zelber-Sagi S, Ivancovsky-Wajcman D, Fliss-Isakov N, Hahn M, Webb M, Shibolet O, Kariv R, Tirosh O. Serum Malondialdehyde is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women. Antioxidants. 2020; 9(7):578. https://doi.org/10.3390/antiox9070578
Chicago/Turabian StyleZelber-Sagi, Shira, Dana Ivancovsky-Wajcman, Naomi Fliss-Isakov, Michal Hahn, Muriel Webb, Oren Shibolet, Revital Kariv, and Oren Tirosh. 2020. "Serum Malondialdehyde is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women" Antioxidants 9, no. 7: 578. https://doi.org/10.3390/antiox9070578