Identification of a New Variety of Avocados (Persea americana Mill. CV. Bacon) with High Vitamin E and Impact of Cold Storage on Tocochromanols Composition
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Samplings
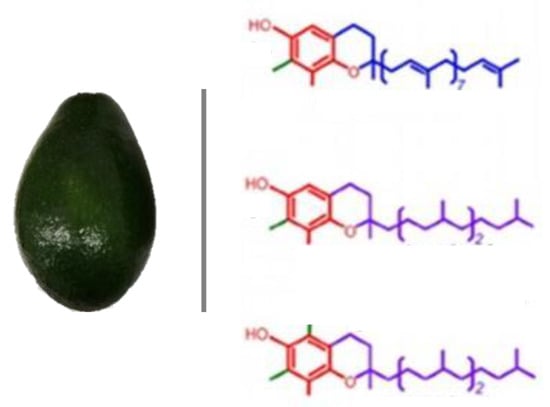
2.2. Tocochromanol Analyses
2.3. Lipid Peroxidation Assays
2.4. Chlorophyll Content
2.5. Statistical Analyses
3. Results
3.1. Identification of Tocochromanols in Various Avocado Varieties
3.2. Cold-induced Changes in Tocochromanol Composition in Bacon Avocados
4. Discussion
4.1. Bacon is a Variety of Avocados with High Tocochromanol Contents
4.2. Effects of Short and Long-Term Storage on Tocochromanol Contents
4.3. Balance between Storage and Nutritional Value
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falk, J.; Munné-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef] [Green Version]
- Kruk, J.; Szymanska, R.; Cela, J.; Munné-Bosch, S. Plastochromanol-8: Fifty years of research. Phytochemistry 2014, 108, 9–16. [Google Scholar] [CrossRef]
- Menné-Saffrané, L.; DellaPena, D. Biosynthesis, regulation and functions of tocochromanols in plants. Plant Physiol. Biochem. 2010, 48, 301–309. [Google Scholar] [CrossRef]
- Kono, N.; Ohto, U.; Hiramatsu, T.; Urabe, M.; Uchida, Y.; Satow, Y.; Arai, H. Impaired a-TTP–PIPs interaction underlies familial vitamin E deficiency. Science 2013, 340, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Cela, J.; Asensi-Fabado, M.; Munné-Bosch, S. Tocotrienols in plants: Occurrence, Biosynthesis and Functions. In Tocotrienols: Vitamin E beyond Tocopherols, 2nd ed.; Tan, B., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Chun, J.; Lee, J.; Ye, L.; Exler, J.; Eitenmiller, R.R. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J. Food Compost. Anal. 2006, 19, 196–204. [Google Scholar] [CrossRef]
- Georgiadou, E.C.; Goulas, V.; Ntourou, T.; Manganaris, G.A.; Kalaitzis, P.; Fotopoulos, V. Regulation of on-tree vitamin E biosynthesis in olive fruit during successive growing years: The impact of fruit development and enviornmental cues. Front. Plant Sci. 2016, 7, 1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trela, A.; Szymanska, R. Less widespread plant oils as a good source of vitamin E. Food Chem. 2019, 296, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Watson, R.R.; Preedy, V.R. Tocotrienols: Vitamin E Beyond Tocopherols; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Kassim, A.; Workneh, T.S.; Bezuidenhout, C.N. A review on postharvest handling of avocado fruit. Afr. J. Agric. Res. 2013, 8, 2385–2402. [Google Scholar] [CrossRef]
- Arpaia, M.L.; Collin, S.; Sievert, J.; Obenland, D. ‘Hass’ avocado quality as influenced by temperature and ethylene prior to and during final ripening. Postharvest Biol. Technol. 2018, 140, 76–84. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and ocygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signalling, acclimation, and practical implications. Antiox. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Leipner, J.; Fracheboud, Y.; Stamp, P. Effect of growing season on the photosynthetic apparatus and leaf antioxidative defenses in two maize genotypes of different chilling tolerance. Environ. Exp. Bot. 1999, 42, 129–139. [Google Scholar] [CrossRef]
- Maeda, H.; Song, W.; Sage, T.L.; DellaPenna, L. Tocopherols play a crucial role in low-temperature adaptation and phloem leading in Arabidopsis. Plant Cell 2006, 18, 1710–1732. [Google Scholar] [CrossRef] [Green Version]
- Gabruk, M.; Habina, I.; Kruk, J.; Dluzewska, J.; Szymanska, R. Natural variation in tocochromanols content in Arabidopsis thaliana accessions – the effect of temperature and light intensity. Physiol. Plant. 2016, 157, 147–160. [Google Scholar] [CrossRef]
- Xiang, N.; Li, C.; Li, G.; Yu, Y.; Hu, J.; Guo, X. Comparative evaluation on vitamin E and carotenoid accumulation in sweet corn (Zea mays L.) seedlings under temperature stress. J. Agric. Food Chem. 2019, 67, 9772–9781. [Google Scholar] [CrossRef]
- Tijero, V.; Teribia, N.; Muñoz, P.; Munné-Bosch, S. Implication of abscisic acid on ripening and quality in sweet cherries: Differential effects during pre- and post-harvest. Front. Plant Sci. 2016, 7, 602. [Google Scholar] [CrossRef] [Green Version]
- Prabath Pathinara, U.A.; Sekozawa, Y.; Sugaya, S.; Gemma, H. Changes in lipid oxidation stability and antioxidant properties of avocado in response to 1-MCP and low oxygen treatment under low-temperature storage. Int. Food Res. J. 2013, 20, 1065–1075. [Google Scholar]
- USDA (US Department of Agriculture). Avocado, almond, pistachio and walnut composition. In Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 24; US Department of Agriculture: Washington, DC, USA, 2011. [Google Scholar]
- Yahia, E.M. Avocado. In Crop Post-Harvest: Science and Technology, 1st ed.; Rees, D., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2013. [Google Scholar]
- FAO: Food and Agriculture Organization of the United Nations. Agriculture Database. 2014. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 February 2020).
- Diaz-Robledo, J. An update of the spanish avocado industry. In Proceedings of Second World Avocado Congress; Lovatt, C.J., Ed.; University of California: Riverside, CA, USA, 1991; pp. 647–651. [Google Scholar]
- Amaral, J.S.; Casal, S.; Torres, D.; Seabra, R.M.; Oliveira, B.P.P. Simultaneous determination of tocopherols and tocotrienols in hazelnuts by a normal phase liquid chromatographic method. Anal. Sci. 2005, 21, 1545–1548. [Google Scholar] [CrossRef] [Green Version]
- Soba, D.; Müller, M.; Aranjuelo IMunné-Bosch, S. Vitamin E in legume nodules: Occurrence and antioxidant function. Phytochemistry 2020, 172, 112261. [Google Scholar] [CrossRef]
- Bou, R.; Codony, R.; Tres, A.; Decker, E.A.; Guardiola, F. Determination of hydroperoxides in foods and biological samples by the ferrous oxidation-xylenol orange method: A review of the factors that influence the method’s performance. Anal. Biochem. 2008, 377, 1–15. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Wang, W.; Bostic, T.R.; Gu, L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010, 122, 1193–1198. [Google Scholar] [CrossRef]
- Vinha, A.F.; Moreira, J.; Barreira, S. Physiochemical parameters, phytochemical composition and antioxidant activity of the algarvian avocado (Persea americana Mill.). J. Agric. Sci. 2013, 5, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Corral-Aguayo, R.D.; Yahia, E.M.; Carrillo-Lopez, A.; González-Aguilar, G. Correlation between some nutritional components and the total antioxidant capacity measured with six different assays in eight horticultural crops. J. Agric. Food Chem. 2008, 56, 10498–10504. [Google Scholar] [CrossRef]
- Lahav, E.; Lavi, U. Avocado: Genetics and breeding. In Breeding Plantation Tree Crops: Tropical Species; Jain, S.M., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Guthrie, N.; Gapor, A.; Chambers, A.F.; Carroll, K.K. Inhibition of proliferation of estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J. Nutr. 1997, 127, 544S–548S. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Mo, H.; Packer, L.; Peterson, D.M. Isolation and identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant, and antitumor properties. J. Agric. Food Chem. 2000, 48, 3130–3140. [Google Scholar] [CrossRef]
- Nakagawa, K.; Shibata, A.; Yamashita, S.; Tsuzuki, T.; Kariya, J.; Oikawa, S.; Miyazawa, T. In vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J. Nutr. 2007, 137, 1938–1943. [Google Scholar] [CrossRef] [Green Version]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [Green Version]
- Thorin, E. Vascular disease risk in patients with hypertriglyceridemia: Endothelial progenitor cells, oxidative stress, accelerated senescence and impaired vascular repair. Can. J. Cardiol. 2011, 27, 538–540. [Google Scholar] [CrossRef]
- Reiner, Z. Hypertriglyceridaemia and risk of coronary arteria disease. Nat. Rev. Cardiol. 2017, 14, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S. Correlation between oxidative stress, nutrition and cancer initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| (A) Tocochromanol (µg/100 g FW) | |||||||
| α-T | β-T | γ-T | γ-TT | δ-TT | PC-8 | Total TCs | |
| Bacon Spain | 2371 ± 148c | 140.3 ± 22.7d | 50.3 ± 11.2 | 42.2 ± 3.3 | 47.7 ± 5.7 | 191 ± 23b | 2848 ± 181c |
| Fuerte Chile | 2190 ± 52bc | 79.8 ± 4.9bcd | 29.9 ± 4.5 | 32.1 ± 5.0 | ND | 192 ± 17b | 2524 ± 53abc |
| Hass Chile | 2068 ± 48bc | 88.4 ± 7.6cd | 51.4 ± 5.1 | 27.4 ± 3.8 | ND | 300 ± 18cd | 2535 ± 61bc |
| Govín Cuba | 2004 ± 129bc | ND | 25.5 ± 5.5 | 29.6 ± 1.8 | ND | 93 ± 4a | 2152 ± 133a |
| Hass Spain | 1997 ± 211bc | 57.9 ± 8.2abc | 50.5 ± 4.3 | 39.2 ± 3.7 | ND | 284 ± 21c | 2434 ± 296abc |
| Hass Perú | 1592 ± 225b | 14.1 ± 8.2a | 36.4 ± 3.4 | 32.1 ± 11.5 | ND | 378 ± 22d | 2062 ± 224ab |
| Hass Brazil | 816 ± 209a | 18.0 ± 6.8ab | 31.8 ± 4.7 | 42.9 ± 6.3 | ND | 226 ± 12b | 1138 ± 217ab |
| (B) Tocochromanol (µg/g DW) | |||||||
| α-T | β-T | γ-T | γ-TT | δ-TT | PC-8 | Total TCs | |
| Bacon Spain | 127.3 ± 10.7c | 7.5 ± 1.2b | 2.7 ± 0.6b | 2.2 ± 0.1bc | 2.5 ± 0.3 | 10.3 ± 1.4b | 153 ± 13b |
| Fuerte Chile | 47.3 ± 4.8ab | 1.7 ± 0.1a | 0.6 ± 0.1a | 0.7 ± 0.1a | ND | 4.2 ± 0.7a | 54 ± 5a |
| Hass Chile | 60.4 ± 1.6ab | 2.6 ± 0.3a | 1.5 ± 0.2ab | 0.8 ± 0.1a | ND | 8.8 ± 0.6ab | 74 ± 2a |
| Govín Cuba | 174.2 ± 16.2d | ND | 2.2 ± 0.4a | 2.6 ± 0.2c | ND | 8.1 ± 0.8ab | 187 ± 17a |
| Hass Spain | 62.3 ± 5.2ab | 1.8 ± 0.2a | 1.6 ± 0.05a | 1.2 ± 0.09ab | ND | 9.0 ± 1.0ab | 76 ± 5a |
| Hass Perú | 78.4 ± 9.8b | 0.7 ± 0.4a | 1.8 ± 0.08a | 1.7 ± 0.6abc | ND | 18.7 ± 0.6c | 102 ± 10a |
| Hass Brazil | 33.5 ± 8.4a | 0.7 ± 0.3a | 1.3 ± 0.2a | 1.8 ± 0.4abc | ND | 9.4 ± 0.5b | 47 ± 8a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincent, C.; Mesa, T.; Munne-Bosch, S. Identification of a New Variety of Avocados (Persea americana Mill. CV. Bacon) with High Vitamin E and Impact of Cold Storage on Tocochromanols Composition. Antioxidants 2020, 9, 403. https://doi.org/10.3390/antiox9050403
Vincent C, Mesa T, Munne-Bosch S. Identification of a New Variety of Avocados (Persea americana Mill. CV. Bacon) with High Vitamin E and Impact of Cold Storage on Tocochromanols Composition. Antioxidants. 2020; 9(5):403. https://doi.org/10.3390/antiox9050403
Chicago/Turabian StyleVincent, Celia, Tania Mesa, and Sergi Munne-Bosch. 2020. "Identification of a New Variety of Avocados (Persea americana Mill. CV. Bacon) with High Vitamin E and Impact of Cold Storage on Tocochromanols Composition" Antioxidants 9, no. 5: 403. https://doi.org/10.3390/antiox9050403