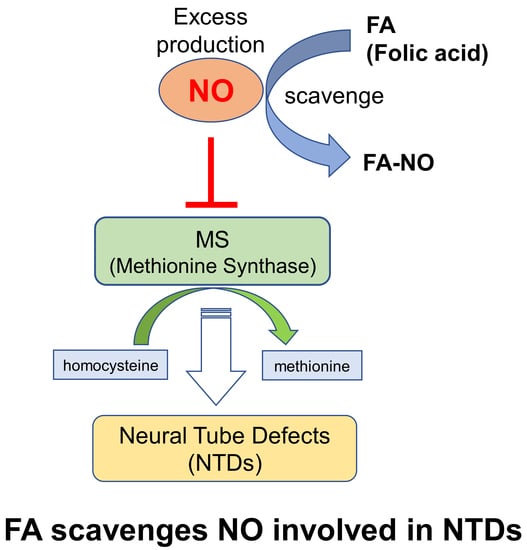
Nitric Oxide Modulation by Folic Acid Fortification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Nitrite Assay on RAW264.7 Macrophages
2.4. iNOS Gene Expression
2.5. Nitric Oxide (NO) Inhibitory Action
2.6. Electron Spin Resonance (ESR) Measurement
2.7. Reaction Product of Folic Acid (FA) and NO
3. Results
3.1. NO Inhibitory Action
3.2. NO Scavenging Activity
3.3. Suppression of iNOS Gene Expression
3.4. Determination of FA-NO by Liquid Chromatography–Mass Spectrometry (LC/MS)
3.5. Products of FA-14NO and FA-15NO
3.6. FA Scavenging NO by ESR Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Czeizel, A.E.; Dudas, I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992, 327, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Cousens, S.; Modell, B.; Lawn, J. Folic acid to reduce neonatal mortality from neural tube disorders. Int. J. Epidemiol. 2010, 39, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenthal, J.; Casas, J.; Taren, D.; Clinton, J.A.; Alina, F.; Jaime, F. Neural tube defects in Latin America and the impact of fortification: A literature review. Public Health Nutr. 2014, 17, 537–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oria, M.; Figueira, R.L.; Scorletti, F.; Sbragia, L.; Owens, K.; Li, Z.; Pathak, B.; Corona, M.U.; Marotta, M.; Encinas, J.L.; et al. CD200-CD200R imbalance correlates with microglia and proinflammatory activation in rat spinal cords exposed to amniotic fluid in retinoic acid-induced spina bifida. Sci. Rep. 2018, 8, 10638–10649. [Google Scholar] [CrossRef]
- Lee, Q.P.; Juchau, M.R. Dysmorphogenic effects of nitric oxide (NO) and NO-synthase inhibition: Studies with intra-amniotic injections of sodium nitroprusside and NG-monomethyl-L-arginine. Teratology 1994, 49, 452–464. [Google Scholar] [CrossRef]
- Danishpajo, I.O.; Gudi, T.; Chen, Y.; Kharitonov, V.G.; Sharma, V.S.; Boss, G.R. Nitric oxide inhibits methionine synthase activity in vivo and disrupts carbon flow through the folate pathway. J. Biol. Chem. 2002, 276, 27296–27303. [Google Scholar] [CrossRef] [Green Version]
- Weil, M.; Abeles, R.; Nachmany, A.; Gold, V.; Michael, E. Folic acid rescues nitric oxide-induced neural tube closure defects. Cell Death Differ. 2004, 11, 361–363. [Google Scholar] [CrossRef] [Green Version]
- Nachmany, A.; Goldmm, V.; Tsur, A.; Arad, D.; Weil, M. Neural tube closure depends on nitric oxide synthase activity. J. Neurochem. 2006, 96, 247–253. [Google Scholar] [CrossRef]
- Taira, J.; Nanbu, H.; Ueda, K. Nitric oxide-scavenging compounds in Agrimonia pilosa Ledeb on LPS-induced RAW264.7 macrophages. Food Chem. 2009, 115, 1221–1227. [Google Scholar] [CrossRef]
- Taira, J.; Misık, V.; Riesz, P. Nitric oxide formation from hydroxylamine by myoglobin and hydrogen peroxide. Biochim. Biophys. Acta 1997, 1336, 502–508. [Google Scholar] [CrossRef]
- Taira, J.; Ohmine, W.; Nanbub, H.; Ueda, K. Inhibition of LPS-stimulated NO production in RAW264.7 macrophages through iNOS suppression and nitrogen radical scavenging by phenolic compounds from Agrimonia pilosa Ledeb. Oxid. Antioxid. Med. Sci. 2013, 2, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Taira, J.; Tsuchida, E.; Katoh, M.C.; Uehara, M.; Ogi, T. Antioxidant capacity of betacyanins as radical scavengers for peroxyl radical and nitric oxide. Food Chem. 2015, 166, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Miyagi, C.; Aniya, Y. Dimerumic acid as an antioxidant from the mold, Monascus anka: The inhibition mechanisms against lipid peroxidation and hemeprotein-mediated oxidation. Biochem. Pharmacol. 2002, 63, 1019–1026. [Google Scholar] [CrossRef]
- Yonetani, T.; Yamamoto, H.; Erman, E.J.; Leigh, S.J., Jr.; Reed, H.G.Ž. Electromagnetic properties of hemoproteins. J. Biol. Chem. 1972, 247, 2447–2455. [Google Scholar]
- Taira, J. Annual Report 2006: Inhibition of Apoptosis in Nitric Oxide Induced PC12 Cells Due to Folic Acid Derivatives; Okinawa Industrial Tech Center: Okinawa, Japan, 2006; Volume 8, pp. 45–51. [Google Scholar]
- Akaike, T.; Yoshida, M.; Miyamoto, Y.; Sato, K.; Kohno, M.; Sasamoto, K.; Miyazaki, K.; Ueda, S.; Maeda, H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.bul.NO (nitric oxide) through a radical reaction. Biochemistry 1993, 32, 827–832. [Google Scholar] [CrossRef]
- Hogg, N.; Singh, R.J.; Joseph, J.; Neese, F.; Kalyanaraman, B. Reactions of nitric oxide with nitronyl nitroxides and oxygen: Prediction of nitrite and nitrate formation. Free Radic. Res. 1995, 22, 47–56. [Google Scholar] [CrossRef]
- Forstermann, U.; Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Stanhewicz, A.E.; Kenney, W.L. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutri. Rev. 2017, 75, 61–70. [Google Scholar] [CrossRef]
- Forstermann, U. Janus-faced role of endothelial NO synthase in vascular disease: Uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol. Chem. 2006, 387, 1521–1533. [Google Scholar] [CrossRef]
- Siragusa, M.; Fleming, I. The eNOS signalosome and its link to endothelial dysfunction. Pflügers Arch. 2016, 468, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- RaviJoshi, J.; Adhikari, S.; Patro, B.S.; Chatrtropadhyay, S.; Mukherjee, T. Free radical scavenging behavior of folic acid: Evidence for possible antioxidant activity. Free Radic. Biol. Med. 2001, 30, 12–1390. [Google Scholar]
- Horvath, C.M. The Jak-STAT pathway stimulated by interferon gamma. Sci. STKE 2004, 2004, tr8. [Google Scholar] [CrossRef] [PubMed]
- Stempelj, M.; Kedinger, M.; Augenlicht, L.; Klampfer, L. Essential role of the JAK/STAT1 signaling pathway in the expression of inducible nitric-oxide synthase in intestinal epithelial cells and its regulation by butyrate. J. Biol. Chem. 2007, 282, 9797–9804. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taira, J.; Ogi, T. Nitric Oxide Modulation by Folic Acid Fortification. Antioxidants 2020, 9, 393. https://doi.org/10.3390/antiox9050393
Taira J, Ogi T. Nitric Oxide Modulation by Folic Acid Fortification. Antioxidants. 2020; 9(5):393. https://doi.org/10.3390/antiox9050393
Chicago/Turabian StyleTaira, Junsei, and Takayuki Ogi. 2020. "Nitric Oxide Modulation by Folic Acid Fortification" Antioxidants 9, no. 5: 393. https://doi.org/10.3390/antiox9050393