Antiproliferative Effect of Bioaccessible Fractions of Four Brassicaceae Microgreens on Human Colon Cancer Cells Linked to Their Phytochemical Composition
Abstract
:1. Introduction
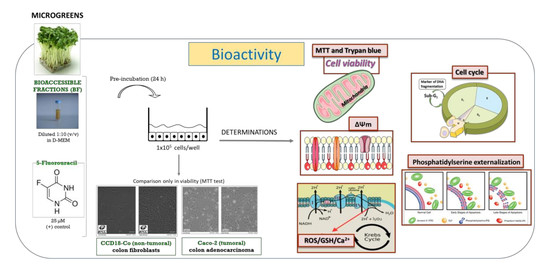
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines and Culture Conditions
2.3. Samples
2.4. Cell Viability Assays
2.4.1. Mitochondrial Enzyme Activity
2.4.2. Trypan Blue Exclusion Test
2.5. Measurement of Mechanisms Involved in Cell Death
2.5.1. Cell Cycle Analysis
2.5.2. Detection of Cellular Apoptosis
2.5.3. Intracellular Reactive Oxygen Species (ROS) Production
2.5.4. Intracellular Glutathione (GSH) Determination
2.5.5. Mitochondrial Membrane Potential Changes (ΔΨM)
2.5.6. Intracellular Calcium (Ca2+) Content
2.6. Statistical Analysis
3. Results and Discussion
3.1. Antiproliferative Effect of Microgreen’s Bioaccessible Fractions on Tumoral Cells
3.2. Cell Cycle Analysis
3.3. Apoptotic Pathway Activation
3.4. Cellular Redox Disruption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Francisco, M.; Tortosa, M.; del Carmen Martínez-Ballesta, M.; Velasco, P.; García-Viguera, C.; Moreno, D.A. Nutritional and phytochemical value of Brassica crops from the agri-food perspective. Ann. Appl. Biol. 2016, 170, 273–285. [Google Scholar] [CrossRef]
- Manchali, S.; Chidambara Murthy, K.N.; Patil, B.N. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Raiola, A.; Errico, A.; Petruk, G.; Monti, D.M.; Barone, A.; Rigano, M.M. Bioactive compounds in Brassicaceae vegetables with role in the prevention of chronic diseases. Molecules 2018, 23, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, U.; Yu, L.L.; Wang, T.T.Y. The science behind microgreens as an exciting new food for the 21st Century. J. Agric. Food Chem. 2018, 66, 1159–11530. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Luu, H.N.; Liu, Y.; Cai, H.; Xu, G.; Shu, X.O. High intake of cruciferous vegetables reduces the risk of gastrointestinal cancers: Results from observational studies. Lancet Gastroenterol. Hepatol. 2019. [Google Scholar] [CrossRef]
- Sanlier, N.; Guler, S.M. The benefits of Brassica vegetables on human health. J. Hum. Health Res. 2018, 1, 104–126. [Google Scholar]
- Tao, J.; Li, Y.; Li, S.; Li, H.B. Plant foods for the prevention and management of colon cancer. J. Funct. Foods 2018, 42, 95–110. [Google Scholar] [CrossRef]
- de la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the bioaccessibility of antioxidant bioactive compounds and minerals of four genotypes of Brassicaceae microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Le, T.N.; Luong, H.Q.; Li, H.P.; Chiu, C.H.; Hsieh, P.C. Broccoli (Brassica oleracea L. var. italica) sprouts as the potential food source for bioactive properties: A comprehensive study on in vitro disease models. Foods 2019, 8, 532. [Google Scholar] [CrossRef] [Green Version]
- Ferrarini, L.; Pellegrini, N.; Mazzeo, T.; Miglio, C.; Galati, S.; Milano, F.; Rossi, C.; Buschini, A. Anti-proliferative activity and chemoprotective effects towards DNA oxidative damage of fresh and cooked Brassicaceae. Br. J. Nutr. 2012, 107, 1324–1332. [Google Scholar] [CrossRef] [Green Version]
- Olsen, H.; Grimmer, S.; Aaby, K.; Saha, S.; Borge, G.I.A. Antiproliferative effects of fresh and thermal processed green and red cultivars of curly kale (Brassica oleracea L. convar. acephala var. sabellica). J. Agric. Food Chem. 2012, 60, 7375–7383. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kim, B.K.; Park, K.Y. Antimutagenic and anticancer effects of leaf mustard and leaf mustard kimchi. Prev. Nutr. Food Sci. 2007, 12, 84–88. [Google Scholar] [CrossRef]
- Kwak, Y.; Lee, J.; Ju, J. Anti-cancer activities of Brassica juncea leaves in vitro. EXCLI J. 2016, 15, 699–710. [Google Scholar] [CrossRef]
- Pocasap, P.; Weerapreeyakul, N.; Barusrux, S. Cancer preventive effect of Thai rat-tailed radish (Raphanus sativus L. var. caudatus Alef). J. Funct. Foods 2013, 5, 1372–1381. [Google Scholar] [CrossRef]
- Cilla, A.; Alegría, A.; Barberá, R.; Lagarda, M.J. Foods or bioactive constituents of food as chemopreventives in cell lines after simulated gastrointestinal digestion: A review. In Oxidative Stress and Chronic Degenerative Diseases. A Role of Antioxidants; Morales-González, J.A., Ed.; IntechOpen: Rijeka, Croatia, 2013; pp. 131–151. [Google Scholar] [CrossRef] [Green Version]
- Ávila-Gálvez, M.A.; González-Sarrías, A.; Espín, J.C. In vitro research on dietary polyphenols and health: A call of caution and guide on how to proceed. J. Agric. Food Chem. 2018, 66, 7857–7858. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Sala, A.; Ávila-Gálvez, M.A.; Cilla, A.; Barberá, R.; García-Llatas, G.; Espín, J.C.; González-Sarrías, A. Physiological concentrations of phytosterols enhance the apoptotic effects of 5-fluorouracil in colon cancer cells. J. Funct. Foods 2018, 49, 52–60. [Google Scholar] [CrossRef]
- López-García, G.; Cilla, A.; Barberá, R.; Alegría, A. Antiproliferative effect of plant sterols at colonic concentrations on Caco-2 cells. J. Funct. Foods 2017, 39, 84–90. [Google Scholar] [CrossRef]
- Cilla, A.; Attanzio, A.; Barberá, R.; Tesoriere, L.; Livrea, M.A. Anti-proliferative effect of main dietary phytosterols and β-cryptoxanthin alone or combined in human colon cancer Caco-2 cells through cytosolic Ca2+ – and oxidative stress induced apoptosis. J. Funct. Foods 2015, 12, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Gómez, L.J.; Gómez, N.A.; Zapata, J.E.; López-García, G.; Cilla, A.; Alegría, A. In-vitro antioxidant capacity and cytoprotective/cytotoxic effects upon Caco-2 cells of red tilapia (Oreochromis spp.) viscera hydrolysates. Food Res. Int. 2019, 120, 52–61. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, A.; Prashant, Y.; Singh, D. Isothiocyanates in Brassica: Potencial anti cancer agents. Asian Pac. J. Cancer Prev. 2016, 17, 4507–4510. [Google Scholar]
- Zhao, Y.; Hu, X.; Zuo, X.; Wang, M. Chemopreventive effects of some popular phytochemicals on human colon cancer: A review. Food Funct. 2018, 9, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Jeżyna, M.; Świeca, M.; Dziki, D.; Baraniak, B.; Czyż, J. Effect of bioaccessibility of phenolic compounds on in vitro anticancer activity of broccoli sprouts. Food Res. Int. 2012, 49, 469–476. [Google Scholar] [CrossRef]
- Li, R.; Song, D.; Vriesekoop, F.; Cheng, L.; Yuang, Q.; Liang, H. Glucoraphenin, sulforaphene, and antiproliferative capacity of radish sprouts in germinating and thermal processes. Eur. Food Res. Technol. 2016, 243, 547–554. [Google Scholar] [CrossRef]
- Diaz-Moralli, S.; Tarrado-Castellarnau, M.; Miranda, A.; Cascante, M. Targeting cell cycle regulation in cancer therapy. Pharmacol. Ther. 2013, 138, 255–271. [Google Scholar] [CrossRef]
- Langner, E.; Lemieszek, M.K.; Rzeski, W. Lycopene, sulforaphane, quercetin, and curcumin applied together show improved antiproliferative potential in colon cancer cells in vitro. J. Food Biochem. 2019, 43, e12802. [Google Scholar] [CrossRef]
- Gamet-Payrastre, L.; Li, P.; Lumeau, S.; Cassar, G.; Dupont, M.A.; Chevolleau, S.; Gasc, N.; Tulliez, J.; Tercé, F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000, 60, 1426–1433. [Google Scholar]
- Chaudhary, A.; Choudhary, S.; Sharma, U.; Vig, A.P.; Arora, S. In vitro evaluation of Brassica sprouts for its antioxidant and antiproliferative potential. Indian J. Pharm. Sci. 2016, 78, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of apoptosis and necrosis by annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb. Protoc. 2016. [Google Scholar] [CrossRef]
- Vejux, A.; Lizard, G. Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Asp. Med. 2009, 30, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Huang, H.; Huang, T.; Wang, Y.; Juan, H. Flow cytometric detection of mitochondrial membrane potential. Bioprotocol 2013, 3, e430. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, A.; Choudhary, S.; Sharma, U.; Vig, A.P.; Singh, B.; Arora, S. Purple head broccoli (Brassica oleracea L. var. italica Plenck), a functional food crop for antioxidant and anticancer potential. J. Food Sci. Technol. 2018, 55, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Nicotera, P.; Orrenius, S. The role of calcium in apoptosis. Cell Calcium 1998, 23, 173–180. [Google Scholar] [CrossRef]
- June, C.H.; Abe, R.; Rabinovitch, P.S. Measurement of intracellular calcium ions by flow cytometry. Curr. Protoc. Cytom. 1997, 2, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawsky, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2017, 8, 15996–16016. [Google Scholar] [CrossRef] [Green Version]
- Varghese, E.; Liskova, A.; Kubatka, P.; Samuel, S.M.; Büsselberg, D. Anti-Angiogenic effects of phytochemicals on miRNA regulating breast cancer progression. Biomolecules 2020, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Abotaleb, M.; Kubatka, P.; Caprnda, M.; Varghese, E.; Zolakova, B.; Zubor, P.; Opatrilova, R.; Kruzliak, P.; Stefanicka, P.; Brüsselberg, D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed. Pharmacother. 2018, 101, 458–477. [Google Scholar] [CrossRef]
- Kapinova, A.; Kubatka, P.; Liskova, A.; Baranenko, D.; Kruzliak, P.; Matta, M.; Brüsselberg, D.; Malicherova, B.; Zulli, A.; Kwon, T.K.; et al. Controlling metastatic cancer: The role of phytochemicals in cell signaling. J. Cancer Res. Clin. Oncol. 2019, 145, 1087–1109. [Google Scholar] [CrossRef]
- Redondo-Blanco, S.; Fernández, J.; Gutiérrez-del-Río, I.; Villar, C.J.; Lombó, F. New insights towards colorectal cancer chemotherapy using natural bioactive compounds. Front. Pharmacol. 2017, 8, 109. [Google Scholar] [CrossRef] [Green Version]




| Bioaccessible Fraction | ||||
|---|---|---|---|---|
| Parameter | Broccoli | Kale | Mustard | Radish |
| Bioactive compound | ||||
| Ascorbic acid (mg/100 g FW) | 0.056 ± 0.04 b | 0.105 ± 0.04 a | 0.114 ± 0.05 a | 0.119 ± 0.04 a |
| Total carotenoids (mg β-carotene/100 g DW) | 0.018 ± 0.01 b | 0.012 ± 0.01 c | 0.025 ± 0.01 a | 0.023 ± 0.02 a |
| Total isothiocyanates (mg sulforaphane/100 g DW) | 20.45 ± 4.79 b | 20.72 ± 1.03 b | 24.89 ± 2.57 b | 51.29 ± 3.39 a |
| Total soluble polyphenols (mg GAE/100 g DW) | 142.79 ± 17.5 a | 144.77 ± 14.01 a | 82.06 ± 3.10 b | 143.48 ± 6.23 a |
| Total antioxidant capacity | ||||
| ORAC (µM Trolox Eq/100 g) | 364.5 ± 28.12 b | 739.15 ± 11.62 a | 745.25 ± 70.16 a | 525.89 ± 72.17 b |
| TEAC (µM Trolox Eq/100 g) | 7.84 ± 0.91 c | 9.87 ± 1.13 b | 11.08 ± 1.86 b | 13.77 ± 1.13 a |
| % Cell Viability | |||
|---|---|---|---|
| MTT Assay | |||
| CCD18-Co | Caco-2 | ||
| Digestion Blank | 102.7 ± 1.7 a,x | 100.9 ± 6.3 a,x | |
| Broccoli | 100.8 ± 5.5 a,x | 90.9 ± 3.8 b,y | |
| Kale | 96.9 ± 3.1 a,x | 89.2 ± 0.5 b,y | |
| Mustard | 86.2 ± 1.2 b,x | 87.5 ± 2.7 b,x | |
| Radish | 94.4 ± 1.3 ab,x | 87.8 ± 2.0 b,y | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuente, B.d.l.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Antiproliferative Effect of Bioaccessible Fractions of Four Brassicaceae Microgreens on Human Colon Cancer Cells Linked to Their Phytochemical Composition. Antioxidants 2020, 9, 368. https://doi.org/10.3390/antiox9050368
Fuente Bdl, López-García G, Máñez V, Alegría A, Barberá R, Cilla A. Antiproliferative Effect of Bioaccessible Fractions of Four Brassicaceae Microgreens on Human Colon Cancer Cells Linked to Their Phytochemical Composition. Antioxidants. 2020; 9(5):368. https://doi.org/10.3390/antiox9050368
Chicago/Turabian StyleFuente, Beatriz de la, Gabriel López-García, Vicent Máñez, Amparo Alegría, Reyes Barberá, and Antonio Cilla. 2020. "Antiproliferative Effect of Bioaccessible Fractions of Four Brassicaceae Microgreens on Human Colon Cancer Cells Linked to Their Phytochemical Composition" Antioxidants 9, no. 5: 368. https://doi.org/10.3390/antiox9050368