Inhibitory Effects of Urtica thunbergiana Ethanol Extract on Atopic Dermatitis-Induced NC/Nga Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. DPPH Scavenging Activity
2.3. Cell Culture, UT Treatment, and Stimulation
2.4. MTT Assay
2.5. Reverse Transcription Polymerase Chain Reaction
2.6. Induction of AD-Like Skin Lesions and Topical Application
2.7. Measurement of Secretion Proteins
2.8. Evaluation of AD-Like Skin Symptoms
2.9. Evaluation of Scratching Behavior
2.10. Measurement of Physiological and Histological Skin Functions
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. Antioxidative Activity of UT
3.2. Effects of UT on Cell Viability and TARC and MDC Production in TNF-α/IFN-γ-Stimulated HaCaT Cells
3.3. Inhibitory Effects of UT on mRNA Expression of Pro-Inflammatory Cytokines and Chemokines in TNF-α/IFN-γ-Stimulated HaCaT Cells
3.4. Inhibitory Effects of UT on NF-κB/STAT1 and MAPKs in TNF-α/IFN-γ-Stimulated HaCaT Cells
3.5. Effect of UT on AD Symptoms on Mouse Skin
3.6. Effect of UT on Histological and Biophysical Characteristics of AD-Induced Skin
3.7. Effect of UT on AD-Related Proteins of NC/Nga Mice Skin
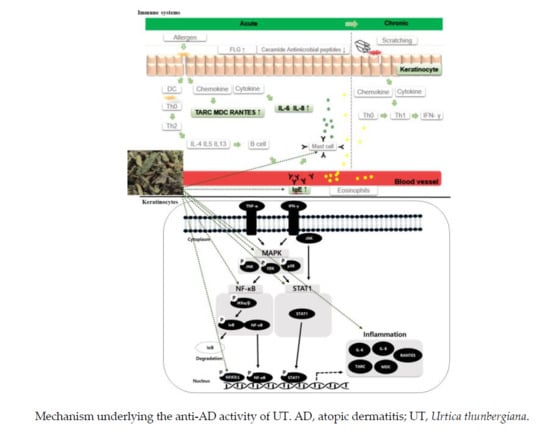
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H.I. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedoszytko, B.; Sokołowska-Wojdyło, M.; Ruckemann-Dziurdzińska, K.; Roszkiewicz, J.; Nowicki, R.J. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Adv. Dermatol. Allergol. Dermatol. Alergol. 2014, 31, 84. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Sayama, K.; Tohyama, M.; Shirakata, Y.; Yang, L.; Hirakawa, S.; Tokumaru, S.; Hashimoto, K. The NF-κB, p38 MAPK and STAT1 pathways differentially regulate the dsRNA-mediated innate immune responses of epidermal keratinocytes. Int. Immunol. 2008, 20, 901–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 2014, 5, 416. [Google Scholar] [CrossRef]
- Allen, B.R. Tacrolimus ointment: Its place in the therapy of atopic dermatitis. J. Allergy Clin. Immunol. 2002, 109, 401–403. [Google Scholar] [CrossRef]
- Azzi, J.R.; Sayegh, M.H.; Mallat, S.G. Calcineurin Inhibitors: 40 Years Later, Can’t Live Without …. J. Immunol. 2013, 191, 5785–5791. [Google Scholar] [CrossRef] [Green Version]
- Pereira, U.; Boulais, N.; Lebonvallet, N.; Pennec, J.P.; Dorange, G.; Misery, L. Mechanisms of the sensory effects of tacrolimus on the skin: Sensory effects of tacrolimus. Br. J. Dermatol. 2010, 163, 70–77. [Google Scholar] [CrossRef]
- Hošková, L.; Málek, I.; Kautzner, J.; Honsová, E.; van Dokkum, R.P.E.; Husková, Z.; Vojtíšková, A.; Varcabová, Š.; Červenka, L.; Kopkan, L. Tacrolimus-induced hypertension and nephrotoxicity in Fawn-Hooded rats are attenuated by dual inhibition of renin–angiotensin system. Hypertens. Res. 2014, 37, 724–732. [Google Scholar] [CrossRef]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.E.; Park, K.H.; Jeong, M.S.; Kim, H.H.; Lee, D.I.; Joo, S.S.; Lee, C.S.; Bang, H.; Choi, Y.W.; Lee, M.K.; et al. Effect of Alnus japonica extract on a model of atopic dermatitis in NC/Nga mice. J. Ethnopharmacol. 2011, 136, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Klooshani, V. Antinociceptive and anti-inflammatory effects of Urtica dioica leaf extract in animal models. Avicenna J. Phytomedicine 2013, 3, 193–200. [Google Scholar]
- Abo-elmatty, D.M.; Essawy, S.S.; Badr, J.M.; Sterner, O. Antioxidant and anti-inflammatory effects of Urtica pilulifera extracts in type 2 diabetic rats. J. Ethnopharmacol. 2013, 145, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Genc, Z.; Yarat, A.; Tunali-Akbay, T.; Sener, G.; Cetinel, S.; Pisiriciler, R.; Caliskan-Ak, E.; Altıntas, A.; Demirci, B. The effect of stinging nettle (Urtica dioica) seed oil on experimental colitis in rats. J. Med. Food 2011, 14, 1554–1561. [Google Scholar] [CrossRef]
- Johnson, T.A.; Sohn, J.; Inman, W.D.; Bjeldanes, L.F.; Rayburn, K. Lipophilic stinging nettle extracts possess potent anti-inflammatory activity, are not cytotoxic and may be superior to traditional tinctures for treating inflammatory disorders. Phytomedicine 2013, 20, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Obertreis, B.; Giller, K.; Teucher, T.; Behnke, B.; Schmitz, H. Anti-inflammatory effect of Urtica dioica folia extract in comparison to caffeic malic acid. Arzneimittel-Forschung 1996, 46, 52–56. [Google Scholar]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.U.H.; Bhat, T.M.; Sharma, P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef]
- Behzadi, A.A.; Kalalian-Moghaddam, H.; Ahmadi, A.H. Effects of Urtica dioica supplementation on blood lipids, hepatic enzymes and nitric oxide levels in type 2 diabetic patients: A double blind, randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 686–695. [Google Scholar]
- Zemmouri, H.; Sekiou, O.; Ammar, S.; El Feki, A.; Bouaziz, M.; Messarah, M.; Boumendjel, A. Urtica dioica attenuates ovalbumin-induced inflammation and lipid peroxidation of lung tissues in rat asthma model. Pharm. Biol. 2017, 55, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Aktas, C.; Erboga, M.; Fidanol Erboga, Z.; Bozdemir Donmez, Y.; Topcu, B.; Gurel, A. Protective effects of Urtica dioica L. on experimental testicular ischaemia reperfusion injury in rats. Andrologia 2017, 49. [Google Scholar] [CrossRef]
- Kanter, M.; Coskun, O.; Budancamanak, M. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J. Gastroenterol. 2005, 11, 6684–6688. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.M.; Bajracharya, A.; Shrestha, A.K. Comparison of nutritional properties of Stinging nettle (Urtica dioica) flour with wheat and barley flours. Food Sci. Nutr. 2016, 4, 119–124. [Google Scholar] [CrossRef]
- Mzid, M.; Ghlissi, Z.; Salem, M.B.; Khedir, S.B.; Chaabouni, K.; Ayedi, F.; Sahnoun, Z.; Hakim, A.; Rebai, T. Chemoprotective role of ethanol extract of Urtica urens L. against the toxicity of imidacloprid on endocrine disruption and ovarian morphometric in female rats, GC/MS analysis. Biomed. Pharmacother. 2018, 97, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, L.L.; Zhang, S.S.; Lu, J.L.; Zeng, Y.; Zhang, H.Y.; Xiang, M. Therapeutic effects of total coumarins from Urtica dentata Hand on collagen-induced arthritis in Balb/c mice. J. Ethnopharmacol. 2011, 138, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; de Souza, P.H.; Behnke, B.; Klingelhoefer, S.; Scheid, A.; Shakibaei, M. Effects of the antirheumatic remedy hox alpha—A new stinging nettle leaf extract—On matrix metalloproteinases in human chondrocytes in vitro. Histol. Histopathol. 2002, 17, 477–485. [Google Scholar] [PubMed]
- Flores-Ocelotl, M.R.; Rosas-Murrieta, N.H.; Moreno, D.A.; Vallejo-Ruiz, V.; Reyes-Leyva, J.; Domínguez, F.; Santos-López, G. Taraxacum officinale and Urtica dioica extracts inhibit dengue virus serotype 2 replication in vitro. BMC Complement. Altern. Med. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Uncini Manganelli, R.E.; Zaccaro, L.; Tomei, P.E. Antiviral activity in vitro of Urtica dioica L., Parietaria diffusa M. et K. and Sambucus nigra L. J. Ethnopharmacol. 2005, 98, 323–327. [Google Scholar] [CrossRef]
- Mzid, M.; Ben Khedir, S.; Ben Salem, M.; Regaieg, W.; Rebai, T. Antioxidant and antimicrobial activities of ethanol and aqueous extracts from Urtica urens. Pharm. Biol. 2017, 55, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Körpe, D.A.; İşerı, Ö.D.; Sahin, F.I.; Cabi, E.; Haberal, M. High-antibacterial activity of Urtica spp. seed extracts on food and plant pathogenic bacteria. Int. J. Food Sci. Nutr. 2013, 64, 355–362. [Google Scholar] [CrossRef]
- Daneshmand, P.; Saliminejad, K.; Dehghan Shasaltaneh, M.; Kamali, K.; Riazi, G.H.; Nazari, R.; Azimzadeh, P.; Khorram Khorshid, H.R. Neuroprotective effects of herbal extract (Rosa canina, Tanacetum vulgare and Urtica dioica) on rat model of sporadic Alzheimer’s disease. Avicenna J. Med. Biotechnol. 2016, 8, 120–125. [Google Scholar]
- Jing, B.; Lv, C.; Li, S.; Fu, M.; Yin, Z. Anti-aging effect of urtica polysaccharides in D-galactose induced aging mice. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2015, 38, 2563–2567. [Google Scholar]
- Hwang, E.; Ngo, H.T.T.; Seo, S.A.; Park, B.; Zhang, M.; Gao, W.; Hoo Yi, T. Urtica thunbergiana prevents UVB-induced premature skin aging by regulating the transcription factor NFATc1: An in vitro and in vivo study. J. Funct. Foods 2017, 36, 162–177. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mansoori, B.; Aghapour, M.; Baradaran, B. Urtica dioica dichloromethane extract induce apoptosis from intrinsic pathway on human prostate cancer cells (PC3). Cell. Mol. Biol. Noisy Gd. Fr. 2016, 62, 78–83. [Google Scholar]
- Gohari, A.; Noorafshan, A.; Akmali, M.; Zamani-Garmsiri, F.; Seghatoleslam, A. Urtica dioica distillate regenerates pancreatic beta cells in Streptozotocin-induced diabetic rats. Iran. J. Med. Sci. 2018, 43, 174–183. [Google Scholar]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef]
- Jeon, Y.D.; Kee, J.Y.; Kim, D.S.; Han, Y.H.; Kim, S.H.; Kim, S.J.; Um, J.Y.; Hong, S.H. Effects of Ixeris dentata water extract and caffeic acid on allergic inflammation in vivo and in vitro. BMC Complement. Altern. Med. 2015, 15, 196. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Longo, R.; Vanella, A. Antioxidant activity of propolis: Role of caffeic acid phenethyl ester and galangin. Fitoterapia 2002, 73 (Suppl. 1), S21–S29. [Google Scholar] [CrossRef]
- Yun, N.; Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef]
- Natarajan, K.; Singh, S.; Burke, T.R.; Grunberger, D.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar] [CrossRef] [Green Version]
- Rajendra Prasad, N.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Shin, H.S.; Satsu, H.; Bae, M.J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.S.M.; Jiao, D.; Chan, B.C.L.; Hon, K.L.; Leung, P.C.; Lau, C.B.S.; Wong, E.C.W.; Cheng, L.; Chan, C.K.M.; Lam, C.W.K.; et al. Anti-Inflammatory activities of pentaherbs formula, Berberine, Gallic Acid and Chlorogenic Acid in Atopic Dermatitis-like skin inflammation. Molecules 2016, 21, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Yang, R.; Zhao, Y.; Liu, C.Z. Separation of chlorogenic acid from honeysuckle crude extracts by macroporous resins. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 867, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against experimental reflux Esophagitis in rats. Biomol. Ther. 2014, 22, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep. 2017, 7, 45723. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, T.; Tsutsui, K.; Nakanishi, E.; Inoue, T.; Hamauzu, Y. Effect of the topical application of an ethanol extract of quince seeds on the development of atopic dermatitis-like symptoms in NC/Nga mice. BMC Complement. Altern. Med. 2017, 17, 80. [Google Scholar] [CrossRef] [Green Version]
- Cha, H.Y.; Ahn, S.; Cheon, J.H.; Park, S.Y.; Kim, K. Hataedock treatment has preventive therapeutic effects for atopic dermatitis through skin barrier protection in Dermatophagoides farinae-induced NC/Nga mice. J. Ethnopharmacol. 2017, 206, 327–336. [Google Scholar] [CrossRef]
- Lim, H.S.; Ha, H.; Lee, M.Y.; Jin, S.E.; Jeong, S.J.; Jeon, W.Y.; Shin, N.R.; Sok, D.E.; Shin, H.K. Saussurea lappa alleviates inflammatory chemokine production in HaCaT cells and house dust mite-induced atopic-like dermatitis in Nc/Nga mice. Food Chem. Toxicol. 2014, 63, 212–220. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, M.J.; Kang, S.; Moon, N.R.; Kim, D.S.; Lee, N.R.; Kim, K.S.; Park, S. Topical treatments of Saussurea costus root and Thuja orientalis L. synergistically alleviate atopic dermatitis-like skin lesions by inhibiting protease-activated receptor-2 and NF-κB signaling in HaCaT cells and Nc/Nga mice. J. Ethnopharmacol. 2017, 199, 97–105. [Google Scholar] [CrossRef]
- Choi, H.; Kim, D.J.; Nam, S.; Lim, S.; Hwang, J.S.; Park, K.S.; Hong, H.S.; Shin, M.K.; Chung, E.; Son, Y. Manifestation of atopic dermatitis-like skin in TNCB-induced NC/Nga mice is ameliorated by topical treatment of substance P, possibly through blockade of allergic inflammation. Exp. Dermatol. 2018, 27, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.M.; Thurston, M.; Omoto, M.; Cherill, R.; Tofte, S.J.; Graeber, M.; Evaluator Group, T.E. The eczema area and severity index (EASI): Assessment of reliability in atopic dermatitis: EASI: Assessment of reliability in AD. Exp. Dermatol. 2001, 10, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, B.K.; Lee, Y.C. Antiasthmatic effects of Hesperidin, a potential Th2 Cytokine antagonist, in a mouse model of allergic asthma. Mediat. Inflamm. 2011, 2011, 485402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.S.; Chun, S.Y.; Lee, M.G.; Kim, S.; Jang, T.J.; Nam, K.S. The prevention of TNF-α/IFN-γ mixture-induced inflammation in human keratinocyte and atopic dermatitis-like skin lesions in Nc/Nga mice by mineral-balanced deep sea water. Biomed. Pharmacother. 2018, 97, 1331–1340. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Chlorogenic acid and its role in biological functions: An up to date. EXCLI J. 2019, 18, 310–316. [Google Scholar]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods. 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Rosa Perez-Gregorio, M.; Simal-Gandara, J. A critical review of the characterization of polyphenol-protein interactions and of their potential use for improving food quality. Curr. Pharm. Des. 2017, 23, 2742–2753. [Google Scholar] [CrossRef]
- Ariza, M.T.; Reboredo-Rodríguez, P.; Cervantes, L.; Soria, C.; Martínez-Ferri, E.; González-Barreiro, C.; Cancho-Grande, B.; Battino, M.; Simal-Gándara, J. Bioaccessibility and potential bioavailability of phenolic compounds from achenes as a new target for strawberry breeding programs. Food Chem. 2018, 248, 155–165. [Google Scholar] [CrossRef]
- Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Pistollato, F.; Zhang, J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, P.; Simal-Gandara, J.; Bompadre, S.; et al. Strawberry tree honey as a new potential functional food. Part 2: Strawberry tree honey increases ROS generation by suppressing Nrf2-ARE and NF-κB signaling pathways and decreases metabolic phenotypes and metastatic activity in colon cancer cells. J. Funct. Foods 2019, 57, 477–487. [Google Scholar] [CrossRef]






| Pharmacological Activity | Animals/Cell Lines | Inducers | Reference PMID * or DOI ** |
|---|---|---|---|
| Anti-inflammatory effect | Swiss mice, Wistar rats | Acetic acid-induced writhing and carrageenan-induced paw edema | 25050274 [12] |
| Albino rats | A high-fat diet and low-dose streptozotocin | 23159471 [13] | |
| Rats | Trinitrobenzene sulfonic acid-induced colitis | 21861725 [14] | |
| Mice | Nicotine-induced damage on sperm parameters, testosterone, and testis tissue | 25071848 [15] | |
| Macrophage immune cells | Lipopolysaccharide treatment | 23092723 [16] | |
| In-vitro assay | Biosynthesis of arachidonic acid metabolites in Rheumatoid arthritis | 8821518 [17] | |
| Wistar rats | Streptozotocin-induced diabetic rats | 23036051 [18] | |
| Human | 50 women with type 2 diabetes | 28078249 [19] | |
| Anti-oxidant effect | Rats | Ovalbumin-induced inflammation | 28385108 [20] |
| Rats | Ischaemia and reperfusion model | 27389487 [21] | |
| Rats | Carbon tetrachloride-treated rats | 16425366 [22] | |
| In-vitro assay | DPPH scavenging activities | 26788318 [23] | |
| Rats | Imidacloprid on endocrine disruption and ovarian morphometric | 29091903 [24] | |
| Anti-arthritic effect | Balb/c mice | Type II collagen-induced arthritis | 22001857 [25] |
| Anti-rheumatic effect | Human chondrocyte cells | IL-1beta treatment | 11962753 [26] |
| Anti-viral effect | BHK-21 cell line | Dengue virus serotype 2 | 29548293 [27] |
| Feline kidney Crandell cells | Feline immunodeficiency virus | 15814267 [28] | |
| Anti-bacterial effect | In vitro assay | DPPH, ABTS, β-carotene, and FRAP scavenging activities | 28084125 [29] |
| Against food spoiling Bacillus pumilus, Shigella spp. and Enterococcus gallinarum, and Clavibacter michiganensis | 23067263 [30] | ||
| Anti-dementia effect | Rats | Sporadic Alzheimer’s disease | 27563424 [31] |
| Anti-aging effect | Mice | D-galactose-induced aging | 27352539 [32] |
| Mice, Fibroblasts | UVB-induced aging | 10.1016/j.jff.2017.07.004 [33] | |
| Anti-cancer effect | Human prostate cancer cells | None | 27064877 [34] |
| Anti-diabetic effect | Sprague-Dawley rats | Streptozotocin-induced diabetes | 29749986 [35] |
| Phytochemical | Structure | Pharmacological Activity | Reference PMID |
|---|---|---|---|
| Caffic acid |  | Anti-inflammation | 23146752 [36] |
| Anti-atopic dermatitis | 26104582 [37] | ||
| Anti-bacteria | 12495706 [38] | ||
| Anti-oxidation | 22209001 [39] | ||
| Immunomodulation | 8799159 [40] | ||
| Anti-proliferation | 21116690 [41] | ||
| Chlorogenic acid |  | Anti-inflammation | 25172696 [42] |
| Anti-atopic dermatitis | 27104513 [43] | ||
| Anti-tumor | 18456581 [44] | ||
| Anti-oxidant | 20933071 [45] | ||
| Immunomodulation | 25414772 [46] | ||
| Anti-viral | 28393840 [47] |
| Gene | Sense | Antisense |
|---|---|---|
| TARC/CCL17 | ATGGCCCCACTGAAGATGCT | TGAACACCAACGGTGGAGGT |
| MDC/CCL22 | AGGACAGAGCATGGCTCGCCTACAGA | TAATGGCAGGGAGGTAGGGCTCCTGA |
| RANTES | CCCCGTGCCCACATCAAGGAGTATTT | CGTCCAGCCTGGGGAAGGTTTTTGTA |
| IL-6 | CTCCTTCTCCACAAGCGCC | GCCGAAGAGCCCTCAGGC |
| IL-8 | TCAGTGCATAAAGACATACTCC | TGGCATCTTCACTGATTCTTG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, H.T.T.; Fang, M.; Hwang, E.; Kim, Y.; Park, B.; Seo, S.A.; Do, N.Q.; Nguyen, Q.T.N.; Yi, T.-H. Inhibitory Effects of Urtica thunbergiana Ethanol Extract on Atopic Dermatitis-Induced NC/Nga Mice. Antioxidants 2020, 9, 197. https://doi.org/10.3390/antiox9030197
Ngo HTT, Fang M, Hwang E, Kim Y, Park B, Seo SA, Do NQ, Nguyen QTN, Yi T-H. Inhibitory Effects of Urtica thunbergiana Ethanol Extract on Atopic Dermatitis-Induced NC/Nga Mice. Antioxidants. 2020; 9(3):197. https://doi.org/10.3390/antiox9030197
Chicago/Turabian StyleNgo, Hien T.T., Minzhe Fang, Eunson Hwang, Yoosung Kim, Bom Park, Seul A Seo, Nhung Quynh Do, Quynh T.N. Nguyen, and Tae-Hoo Yi. 2020. "Inhibitory Effects of Urtica thunbergiana Ethanol Extract on Atopic Dermatitis-Induced NC/Nga Mice" Antioxidants 9, no. 3: 197. https://doi.org/10.3390/antiox9030197