Age-Dependent Vulnerability to Oxidative Stress of Postnatal Rat Pyramidal Motor Cortex Neurons
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Animals and Preparation of the Brain Slices
2.2. Whole-Cell Patch Clamp Recordings and Analysis
2.3. Drugs and General Protocol
2.4. Current- and Voltage-Clamp Recordings
2.5. Measurement of Lipid Peroxidation
2.6. Measurement of GSH and other Non-Protein Free Thiols in Brain Slices
2.7. Measurement of the Glutathione Reductase (GR) Activity
3. Statistical Analysis
4. Results
4.1. CH Does Not Affect the Intrinsic Membrane Properties of Newborn Rats
4.2. CH Does Affect the Intrinsic Membrane Properties of Infantile Rats
4.3. CH Abolishes a Tonic GABAergic Current Mediated by GABAA Receptors
4.4. Exposure to CH Induced Lipid Peroxidation
4.5. CH Treatment Rapidly Increased the Free Thiol Content in Newborn Rats
4.6. CH Activates Glutathione Reductase in Newborn Rats after Treatment Initiation
4.7. Prevention of Membrane Excitability Changes Caused by Cumene Hydroperoxide by Glutathione Monoethyl Ester
5. Discussion
5.1. CH as a Model to Study the Effects of Oxidative Stress
5.2. Influence of CH on the Passive and Active Membrane Properties
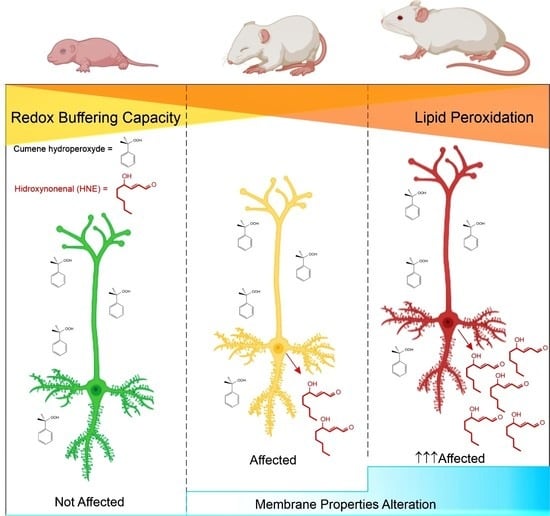
5.3. Age-Dependent Vulnerability to Oxidative Stress
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Reactive oxygen species, oxidative damage and cell death. In Immunity Inflammation in Health and Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 45–55. [Google Scholar]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: A narrative review. Adv. Ther. 2019, 37, 113–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Gluba-Brzózka, A. Oxidative stress in ESRD patients on dialysis and the risk of cardiovascular diseases. Antioxidants 2020, 9, 1079. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The role of oxidative stress in physiopathology and pharmacological treatment with pro- and antioxidant properties in chronic diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Ragagnin, A.M.G.; Shadfar, S.; Vidal, M.; Jamali, M.S.; Atkin, J.D. Motor neuron susceptibility in ALS/FTD. Front. Neurosci. 2019, 13, 532. [Google Scholar] [CrossRef] [Green Version]
- Obrador, E.; Salvador, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. Oxidative stress, neuroinflammation and mitochondria in the pathophysiology of amyotrophic lateral sclerosis. Antioxidants 2020, 9, 901. [Google Scholar] [CrossRef]
- Jovanovic, Z.; Jovanovic, S. Comparison of the effects of cumene hydroperoxide and hydrogen peroxide on Retzius nerve cells of the leech Haemopis sanguisuga. Exp. Anim. 2013, 62, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Leirós, M.; Alonso, E.; Rateb, M.E.; Houssen, W.E.; Ebel, R.; Jaspars, M.; Alfonso, A.; Botana, L.M. Bromoalkaloids protect primary cortical neurons from induced oxidative stress. ACS Chem. Neurosci. 2015, 6, 331–338. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Pardillo-Díaz, R.; Carrascal, L.; Ayala, A.; Nunez-Abades, P. Oxidative stress induced by cumene hydroperoxide evokes changes in neuronal excitability of rat motor cortex neurons. Neuroscience 2015, 289, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, L.; Nieto-Gonzalez, J.L.; Núñez-Abades, P.; Torres, B. Temporal sequence of changes in electrophysiological properties of oculomotor motoneurons during postnatal development. Neuroscience 2006, 140, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, L.; Luque, M.A.; Sobrino, V.; Torres, B.; Nunez-Abades, P. Postnatal development enhances the effects of cholinergic inputs on recruitment threshold and firing rate of rat oculomotor nucleus motoneurons. Neuroscience 2010, 171, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, L.; Nieto-González, J.L.; Torres, B.; Nunez-Abades, P. Diminution of voltage threshold plays a key role in determining recruitment of oculomotor nucleus motoneurons during postnatal development. PLoS ONE 2011, 6, E28748. [Google Scholar] [CrossRef] [PubMed]
- Pardillo-Díaz, R.; Carrascal, L.; Muñoz, M.F.; Ayala, A.; Nunez-Abades, P. Time and dose dependent effects of oxidative stress induced by cumene hydroperoxide in neuronal excitability of rat motor cortex neurons. Neurotoxicology 2016, 53, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Segev, A.; Garcia-Oscos, F.; Kourrich, S. Whole-cell patch-clamp recordings in brain slices. J. Vis. Exp. 2016, 112, E54024. [Google Scholar] [CrossRef]
- Torres-Torrelo, J.; Rodriguez-Rosell, D.; Nunez-Abades, P.; Carrascal, L.; Torres, B. Glutamate modulates the firing rate in oculomotor nucleus motoneurons as a function of the recruitment threshold current. J. Physiol. 2012, 590, 3113–3127. [Google Scholar] [CrossRef]
- Nieto-Gonzalez, J.L.; Carrascal, L.; Nunez-Abades, P.; Torres, B. Phasic and tonic firing properties in rat oculomotor nucleus motoneurons, studied in vitro. Eur. J. Neurosci. 2007, 25, 2682–2696. [Google Scholar] [CrossRef]
- Nieto-Gonzalez, J.L.; Carrascal, L.; Nunez-Abades, P.; Torres, B. Muscarinic modulation of recruitment threshold and firing rate in rat oculomotor nucleus motoneurons. J. Neurophysiol. 2009, 101, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Torres-Torrelo, J.; Torres, B.; Carrascal, L. Modulation of the input-output function by GABAA receptor-mediated currents in rat oculomotor nucleus motoneurons. J. Physiol. 2014, 592, 5047–5064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrales, F.J.; Ruiz, F.; Mato, J.M. In vivo regulation by glutathione of methionine adenosyltransferase S-nitrosylation in rat liver. J. Hepatol. 1999, 31, 887–894. [Google Scholar] [CrossRef] [Green Version]
- Castro, C.; Millian, N.S.; Garrow, T.A. Liver betaine-homocysteine S-methyltransferase activity undergoes a redox switch at the active site zinc. Arch. Biochem. Biophys. 2008, 472, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabaneda, L.G.; Geribaldi-Doldán, N.; Murillo-Carretero, M.; Carrasco, M.; Martínez-Salas, J.M.; Verástegui, C.; Castro, C. Altered regulation of the Spry2/Dyrk1A/PP2A triad by homocysteine impairs neural progenitor cell proliferation. Biochim. Biophys. Acta 2016, 1863, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M.; Rabaneda, L.G.; Murillo-Carretero, M.; Ortega-Martínez, S.; Martínez-Chantar, M.L.; Woodhoo, A.; Luka, Z.; Wagner, C.; Lu, S.C.; Mato, J.M.; et al. Glycine N-methyltransferase expression in the hippocampus and its role in neurogenesis and cognitive performance. Hippocampus 2014, 24, 840–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardillo-Diaz, R.; Carrascal, L.; Barrionuevo, G.; Nunez-Abades, P. Oxidative stress induced by cumene hydroperoxide produces synaptic depression and transient hyperexcitability in rat primary motor cortex neurons. Mol. Cell. Neurosci. 2017, 82, 204–217. [Google Scholar] [CrossRef]
- Klein, J.A.; Ackerman, S.L. Oxidative stress, cell cycle, and neurodegeneration. J. Clin. Investig. 2003, 111, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Vimard, F.; Saucet, M.; Nicole, O.; Feuilloley, M.; Duval, D. Toxicity induced by cumene hydroperoxide in PC12 cells: Protective role of thiol donors. J. Biochem. Mol. Toxicol. 2011, 25, 205–215. [Google Scholar] [CrossRef]
- Vimard, F.; Nouvelot, A.; Duval, D. Cytotoxic effects of an oxidative stress on neuronal-like pheochromocytoma cells (PC12). Biochem. Pharmacol. 1996, 51, 1389–1395. [Google Scholar] [CrossRef]
- Nakaya, H.; Takeda, Y.; Tohse, N.; Kanno, M. Mechanism of the membrane depolarization induced by oxidative stress in guinea-pig ventricular cells. J. Mol. Cell. Cardiol. 1992, 24, 523–534. [Google Scholar] [CrossRef]
- Sakmann, B.; Trube, G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J. Physiol. 1984, 347, 659–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, N.D.; Nistri, A. Substance P and TRH share a common effector pathway in rat spinal motoneurones: An in vitro electrophysiological investigation. Neurosci. Lett. 1993, 153, 115–119. [Google Scholar] [CrossRef]
- Nani, F.; Cifra, A.; Nistri, A. Transient oxidative stress evokes early changes in the functional properties of neonatal rat hypoglossal motoneurons in vitro. Eur. J. Neurosci. 2010, 31, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Dantzler, H.A.; Matott, M.P.; Martinez, D.; Kline, D.D. Hydrogen peroxide inhibits neurons in the paraventricular nucleus of the hypothalamus via potassium channel activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, M.; Hirano, T.; Watanabe, K.; Shoji, H.; Ohashi, N.; Baba, H.; Endo, N.; Kohno, T. Hydrogen peroxide modulates neuronal excitability and membrane properties in ventral horn neurons of the rat spinal cord. Neuroscience 2016, 331, 206–220. [Google Scholar] [CrossRef]
- Frantseva, M.V.; Perez Velazquez, J.L.; Carlen, P.L. Changes in membrane and synaptic properties of thalamocortical circuitry caused by hydrogen peroxide. J. Neurophysiol. 1998, 80, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Zanette, G.; Tamburin, S.; Manganotti, P.; Refatti, N.; Forgione, A.; Rizzuto, N. Different mechanisms contribute to motor cortex hyperexcitability in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2002, 113, 1688–1697. [Google Scholar] [CrossRef]
- Ziemann, U.; Winter, M.; Reimers, C.D.; Reimers, K.; Tergau, F.; Paulus, W. Impaired motor cortex inhibition in patients with amyotrophic lateral sclerosis. Evidence from paired transcranial magnetic stimulation. Neurology 1997, 49, 1292–1298. [Google Scholar] [CrossRef]
- Sebe, J.Y.; Looke-Stewart, E.C.; Estrada, R.C.; Baraban, S.C. Robust tonic GABA currents can inhibit cell firing in mouse newborn neocortical pyramidal cells. Eur. J. Neurosci. 2010, 32, 1310–1318. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Gonzalez, J.L.; Moser, J.; Lauritzen, M.; Schmitt-John, T.; Jensen, K. Reduced GABAergic inhibition explains cortical hyperexcitability in the wobbler mouse model of ALS. Cereb. Cortex 2011, 21, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Pouokam, E.; Rehn, M.; Diener, M. Effects of H2O2 at rat myenteric neurones in culture. Eur. J. Pharmacol. 2009, 615, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.-F.; Ouyang, S.; Zhang, H. The characteristics and oxidative modulation of large-conductance calcium-activated potassium channels in guinea-pig colon smooth muscle cells. Acta Physiol. Sin. 2009, 61, 285–291. [Google Scholar]
- Hasan, S.M.; Joe, M.; Alshuaib, W.B. Oxidative stress alters physiological and morphological neuronal properties. Neurochem. Res. 2007, 32, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.M.K.; Redzic, Z.B.; Alshuaib, W.B. Hydrogen peroxide-induced reduction of delayed rectifier potassium current in hippocampal neurons involves oxidation of sulfhydryl groups. Brain Res. 2013, 1520, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, Z.D.; Stanojevic, M.B.; Nedeljkov, V.B. The neurotoxic effects of hydrogen peroxide and copper in Retzius nerve cells of the leech Haemopis sanguisuga. Biol. Open 2016, 5, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Cabungcal, J.H.; Counotte, D.S.; Lewis, E.; Tejeda, H.A.; Piantadosi, P.; Pollock, C.; Calhoon, G.G.; Sullivan, E.; Presgraves, E.; Kil, J.; et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron 2014, 83, 1073–1084. [Google Scholar] [CrossRef] [Green Version]
- Carrascal, L.; Nunez-Abades, P.; Ayala, A.; Cano, M. Role of melatonin in the inflammatory process and its therapeutic potential. Curr. Pharm. Des. 2018, 24, 1563–1588. [Google Scholar] [CrossRef]
- Park, H.-A.; Ellis, A.C. Dietary antioxidants and Parkinson’s disease. Antioxidants 2020, 9, 570. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [Green Version]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Mehrabadi, S.; Sadr, S.S. Administration of Vitamin D3 and E supplements reduces neuronal loss and oxidative stress in a model of rats with Alzheimer’s disease. Neurol. Res. 2020, 42, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, Y.J.; Lee, E.K.; Park, S.W.; Yu, H.G. Antioxidative effects of ascorbic acid and astaxanthin on arpe-19 cells in an oxidative stress model. Antioxidants 2020, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, D.L.; Doorn, J.A.; Kiebler, Z.; Petersen, D.R. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem. Res. Toxicol. 2005, 18, 1324–1331. [Google Scholar] [CrossRef]
- Deepashree, S.; Niveditha, S.; Shivanandappa, T.; Ramesh, S.R. Oxidative stress resistance as a factor in aging: Evidence from an extended longevity phenotype of Drosophila melanogaster. Biogerontology 2019, 20, 497–513. [Google Scholar] [CrossRef]
- Guevara, R.; Gianotti, M.; Oliver, J.; Roca, P. Age and sex-related changes in rat brain mitochondrial oxidative status. Exp. Gerontol. 2011, 46, 923–928. [Google Scholar] [CrossRef]
- Cassarino, D.S.; Bennett, J.P. An evaluation of the role of mitochondria in neurodegenerative diseases: Mitochondrial mutations and oxidative pathology, protective nuclear responses, and cell death in neurodegeneration. Brain Res. Rev. 1999, 29, 1–25. [Google Scholar] [CrossRef]
- Zou, S.; Meadows, S.; Sharp, L.; Jan, L.Y.; Jan, Y.N. Genome-wide study of aging and oxidative stress response in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 13726–13731. [Google Scholar] [CrossRef] [Green Version]
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; d’Angelo, M. Neuronal cells rearrangement during aging and neurodegenerative disease: Metabolism, oxidative stress and organelles dynamic. Front. Mol. Neurosci. 2019, 12, 132. [Google Scholar] [CrossRef] [Green Version]
- Porcellotti, S.; Fanelli, F.; Fracassi, A.; Sepe, S.; Cecconi, F.; Bernardi, C.; Cimini, A.; Cerù, M.P.; Moreno, S. Oxidative stress during the progression of β-amyloid pathology in the neocortex of the Tg2576 mouse model of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2015, 2015, 967203. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Peana, M.; Maes, M.; Dadar, M.; Severin, B. The glutathione system in Parkinson’s disease and its progression. Neurosci. Biobehav. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kussmaul, L.; Hamprecht, B.; Dringen, R. The detoxification of cumene hydroperoxide by the glutathione system of cultured astroglial cells hinges on hexose availability for the regeneration of NADPH. J. Neurochem. 1999, 73, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Pawlowski, P.G.; Hirrlinger, J. Peroxide detoxification by brain cells. J. Neurosci. Res. 2005, 79, 157–165. [Google Scholar] [CrossRef] [PubMed]





| Membrane Properties | Control | CH 5 min | CH 15 min | CH 30 min |
|---|---|---|---|---|
| Membrane potential (mV) | −64.1 ± 1.7 | −63.9 ± 2.0 | −64.4 ± 2.2 | −63.5 ± 2.4 |
| Input resistance (MΩ) | 744.6 ± 46.1 | 710.5 ± 50.5 | 675.3 ± 55.1 | 665.8 ± 49.3 |
| Rheobase (pA) | 34.7 ± 4.9 | 36.0 ± 4.0 | 40.3 ± 4.9 | 42.7 ± 6.7 |
| Voltage depolarization (mV) | 21.7 ± 2.0 | 21.1 ± 1.6 | 23.5 ± 2.1 | 22.77 ± 2.2 |
| Voltage threshold (mV) | −43.5 ± 2.8 | −44.5 ± 2.6 | −42.3 ± 2.3 | −41.5 ± 2.4 |
| Action potential amplitude (mV) | 92.4 ± 1.0 | 91.1 ± 1.6 | 91.3 ± 1.7 | 89.7 ± 2.0 |
| Action potential duration (ms) | 2.98 ±0.16 | 3.03 ±0.18 | 2.95 ±0.18 | 3.01 ±0.22 |
| Gain (AP·s−1·pA−1) | 91.1 ± 6.4 | 90.0 ± 7.4 | 93.3 ± 9.1 | 85.6 ± 8.8 |
| Maximum frequency (AP·s−1) | 18.1 ± 1.4 | 18.6 ± 2.0 | 17.8 ± 2.0 | 16.7 ± 1.4 |
| Cancellation current (pA) | 174.2 ± 17.2 | 192.9 ± 14.7 | 186.2 ± 15.9 | 173.3 ± 17.3 |
| Membrane Properties | Control | CH 5 min | CH 15 min | CH 30 min |
|---|---|---|---|---|
| Membrane potential (mV) | −64.5 ± 1.4 | −63.5 ± 1.5 | −58.3 ± 1.1 *+ | −57.4 ± 1.5 * |
| Input resistance (MΩ) | 285.4 ± 12.7 | 302.2 ± 13.3 | 348.0 ± 15.5 + | 412.5 ± 29.8 *+ |
| Rheobase (pA) | 110.0 ± 14.7 | 93.0 ± 11.2 | 87.0 ± 11.0 + | 72.0 ± 9.6 *+ |
| Voltage depolarization (mV) | 23.9 ± 1.5 | 23.1 ± 1.3 | 21.4 ± 1.7 | 20.9 ± 1.9 |
| Voltage threshold (mV) | −42.0 ± 1.1 | −41.2 ± 1.0 | −38.7 ± 1.5 | −37.9 ± 2.2 |
| Action potential amplitud (mV) | 104.1 ± 2.9 | 100.4 ± 3.1 | 98.5 ± 2.8 | 97.6 ± 2.3 |
| Action potential duration (ms) | 1.96 ± 0.11 | 2.15 ± 0.11 | 2.13 ± 0.11 | 2.04 ± 0.10 |
| Gain (AP·s−1·pA−1) | 53.8 ± 7.0 | 49.9 ± 7.1 | 57.6 ± 9.7 | 60.7 ± 11.1 |
| Maximum frequency (AP·s−1) | 20.6 ± 2.3 | 20.2 ± 2.4 | 18.7 ± 2.3 | 17.5 ± 2.0 * |
| Cancellation current (pA) | 437.0 ± 64.6 | 457.0 ± 71.7 | 345.0 ± 48.9 *+ | 302.0 ± 42.6 *+ |
| Membrane Properties | Alteration (Minimum Incubation Time Required) | ||
|---|---|---|---|
| Newborn | Infantile | Young Adult | |
| Membrane potential | = | ↑ (15 min) | ↑ (5 min) |
| Input resistance | = | ↑ (15 min) | ↑ (5 min) ↓ (15 min) |
| Rheobase | = | ↓ (15 min) | ↓ (5 min) ↑ (15 min) |
| Voltage depolarization | = | = | ↓ (5 min) |
| Voltage threshold | = | = | = |
| Action potential amplitude | = | = | ↓ (5 min) |
| Action potential duration | = | = | ↑ (5 min) |
| Gain | = | = | ↓ (15 min) |
| Maximum frequency | = | ↓ (30 min) | ↓ (5 min) |
| Cancellation current | = | ↓ (15 min) | ↓ (15 min) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrascal, L.; Gorton, E.; Pardillo-Díaz, R.; Perez-García, P.; Gómez-Oliva, R.; Castro, C.; Nunez-Abades, P. Age-Dependent Vulnerability to Oxidative Stress of Postnatal Rat Pyramidal Motor Cortex Neurons. Antioxidants 2020, 9, 1307. https://doi.org/10.3390/antiox9121307
Carrascal L, Gorton E, Pardillo-Díaz R, Perez-García P, Gómez-Oliva R, Castro C, Nunez-Abades P. Age-Dependent Vulnerability to Oxidative Stress of Postnatal Rat Pyramidal Motor Cortex Neurons. Antioxidants. 2020; 9(12):1307. https://doi.org/10.3390/antiox9121307
Chicago/Turabian StyleCarrascal, Livia, Ella Gorton, Ricardo Pardillo-Díaz, Patricia Perez-García, Ricardo Gómez-Oliva, Carmen Castro, and Pedro Nunez-Abades. 2020. "Age-Dependent Vulnerability to Oxidative Stress of Postnatal Rat Pyramidal Motor Cortex Neurons" Antioxidants 9, no. 12: 1307. https://doi.org/10.3390/antiox9121307