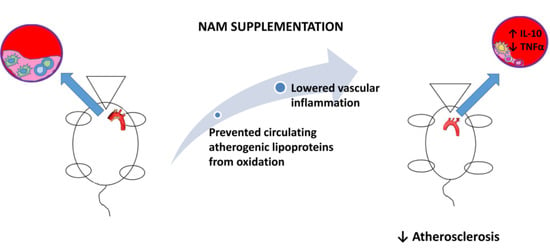
Nicotinamide Prevents Apolipoprotein B-Containing Lipoprotein Oxidation, Inflammation and Atherosclerosis in Apolipoprotein E-Deficient Mice
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Effect of NAM on Gross Parameters and Systemic Phenotype
3.2. NAM Administration Prevents the Development of Aortic Atherosclerosis
3.3. NAM Administration Directly Protects against the Oxidation of Non-HDL Lipoproteins
3.4. The NAM Treatment Improves Plasma and Aortic Inflammation
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piche, M.E.; Poirier, P.; Lemieux, I.; Despres, J.P. Overview of Epidemiology and Contribution of Obesity and Body Fat Distribution to Cardiovascular Disease: An Update. Prog. Cardiovasc. Dis. 2018, 61, 103–113. [Google Scholar] [CrossRef] [PubMed]
- McGill, H.C., Jr. Fatty streaks in the coronary arteries and aorta. Lab. Investig. 1968, 18, 560–564. [Google Scholar] [PubMed]
- Geer, J.C.; McGill, H.C., Jr.; Robertson, W.B.; Strong, J.P. Histologic characteristics of coronary artery fatty streaks. Lab. Investig. 1968, 18, 565–570. [Google Scholar] [PubMed]
- Skalen, K.; Gustafsson, M.; Rydberg, E.K.; Hulten, L.M.; Wiklund, O.; Innerarity, T.L.; Boren, J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 2002, 417, 750–754. [Google Scholar] [CrossRef]
- Sampson, U.K.; Fazio, S.; Linton, M.F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: The evidence, etiology, and therapeutic challenges. Curr. Atheroscler. Rep. 2012, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.J.; Villines, T.C.; Stanek, E.J.; Devine, P.J.; Griffen, L.; Miller, M.; Weissman, N.J.; Turco, M. Extended-release niacin or ezetimibe and carotid intima-media thickness. N. Engl. J. Med. 2009, 361, 2113–2122. [Google Scholar] [CrossRef] [Green Version]
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, R.; Joyal, S.V.; Hill, K.A.; Pfeffer, M.A.; Skene, A.M. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004, 350, 1495–1504. [Google Scholar] [CrossRef]
- Guthrie, R. Niacin or ezetimibe for patients with, or at risk of coronary heart disease. Clin. Med. Insights Cardiol. 2010, 4, 99–110. [Google Scholar] [CrossRef]
- Holzhauser, E.; Albrecht, C.; Zhou, Q.; Buttler, A.; Preusch, M.R.; Blessing, E.; Katus, H.A.; Bea, F. Nicotinic acid has anti-atherogenic and anti-inflammatory properties on advanced atherosclerotic lesions independent of its lipid-modifying capabilities. J. Cardiovasc. Pharmacol. 2011, 57, 447–454. [Google Scholar] [CrossRef]
- Lipton, P.; Michels, J.G. The Effects of Nicotinic Acid on Rabbit Hypercholesterolemia and Atherogenesis. Geriatrics 1965, 71, 379–387. [Google Scholar]
- Lukasova, M.; Hanson, J.; Tunaru, S.; Offermanns, S. Nicotinic acid (niacin): New lipid-independent mechanisms of action and therapeutic potentials. Trends Pharmacol. Sci. 2011, 32, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Orbetsova, V.; Iurukova, T.; Kirov, T. Regression of experimental cholesterol-induced atherosclerosis in rabbits after stopping the atherogenic diet and after treatment with nicotinic acid. Eksp. Med. Morfol. 1985, 24, 32–38. [Google Scholar]
- Strack, A.M.; Carballo-Jane, E.; Wang, S.P.; Xue, J.; Ping, X.; McNamara, L.A.; Thankappan, A.; Price, O.; Wolff, M.; Wu, T.J.; et al. Nicotinic acid and DP1 blockade: Studies in mouse models of atherosclerosis. J. Lipid Res. 2013, 54, 177–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, F.; Beja, M.L.; Serra e Silva, P.; Cabrita, S.; Franca, M.B.; Almeida, L.; Morgadinho, M.T. Experimental atherogenesis and vascular noradrenaline content in NZW rabbits and activity of a new nicotinic acid derivative (L 44-0). J. Lipid Mediat. 1991, 3, 167–175. [Google Scholar] [PubMed]
- Kuhnast, S.; Louwe, M.C.; Heemskerk, M.M.; Pieterman, E.J.; van Klinken, J.B.; van den Berg, S.A.; Smit, J.W.; Havekes, L.M.; Rensen, P.C.; van der Hoorn, J.W.; et al. Niacin Reduces Atherosclerosis Development in APOE*3Leiden. CETP Mice Mainly by Reducing NonHDL-Cholesterol. PLoS ONE 2013, 8, e66467. [Google Scholar] [CrossRef] [Green Version]
- Lukasova, M.; Malaval, C.; Gille, A.; Kero, J.; Offermanns, S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Investig. 2011, 121, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.J.; Yan, L.; Charlton, F.; Witting, P.; Barter, P.J.; Rye, K.A. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 968–975. [Google Scholar] [CrossRef] [Green Version]
- Parwaresch, M.R.; Haacke, H.; Mader, C. Efficacy of hypolipidemic treatment in inhibition of experimental atherosclerosis: The effect of nicotinic acid and related compounds. Atherosclerosis 1978, 31, 395–401. [Google Scholar] [CrossRef]
- Ost, C.R.; Stenson, S. Regression of peripheral atherosclerosis during therapy with high doses of nicotinic acid. Scand. J. Clin. Lab. Investig. 1967, 99, 241–245. [Google Scholar]
- Superko, H.R.; Zhao, X.Q.; Hodis, H.N.; Guyton, J.R. Niacin and heart disease prevention: Engraving its tombstone is a mistake. J. Clin. Lipidol. 2017, 11, 1309–1317. [Google Scholar] [CrossRef] [Green Version]
- Kiss, T.; Giles, C.B.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Gautam, T.; Csipo, T.; Nyul-Toth, A.; Lipecz, A.; Szabo, C.; et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience 2019, 41, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Hawrylyshyn, K.M. Nicotinamide Riboside Delivery Gener y Generates NAD+ Reserves to Protect Vascular Cells against Oxidative Damage. Ph.D. Thesis, Graduate Program in Biochemistry, The University of Western Ontario, London, ON, Canada, 2015. Available online: https://ir.lib.uwo.ca/etd/2891 (accessed on 22 June 2015).
- De Picciotto, N.E.; Gano, L.B.; Johnson, L.C.; Martens, C.R.; Sindler, A.L.; Mills, K.F.; Imai, S.; Seals, D.R. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 2016, 15, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Huang, G.X.; Bonkowski, M.S.; Longchamp, A.; Li, C.; Schultz, M.B.; Kim, L.J.; Osborne, B.; Joshi, S.; Lu, Y.; et al. Impairment of an Endothelial NAD(+)-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2018, 173, 74–89.e20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, J.; Baur, J.A.; Imai, S.I. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waetzig, G.; Seegert, D. Pharmaceutical Composition Containing Nicotinic Acid, Nicotinamide, and/or Tryptophan for Positively Influencing the Intestinal Microbiota. 2015. Available online: https://patents.google.com/patent/EP2861229A1/en (accessed on 22 April 2015).
- Shalita, A.R.; Smith, J.G.; Parish, L.C.; Sofman, M.S.; Chalker, D.K. Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne vulgaris. Int. J. Dermatol. 1995, 34, 434–437. [Google Scholar] [CrossRef]
- Emanuele, E.; Bertona, M.; Altabas, K.; Altabas, V.; Alessandrini, G. Anti-inflammatory effects of a topical preparation containing nicotinamide, retinol, and 7-dehydrocholesterol in patients with acne: A gene expression study. Clin. Cosmet. Investig. Dermatol. 2012, 5, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Fabbrocini, G.; Cantelli, M.; Monfrecola, G. Topical nicotinamide for seborrheic dermatitis: An open randomized study. J. Dermatol. Treat. 2014, 25, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Ashkani Esfahani, S.; Khoshneviszadeh, M.; Namazi, M.R.; Noorafshan, A.; Geramizadeh, B.; Nadimi, E.; Razavipour, S.T. Topical Nicotinamide Improves Tissue Regeneration in Excisional Full-Thickness Skin Wounds: A Stereological and Pathological Study. Trauma Mon. 2015, 20, e18193. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, B.M.; Blakemore, W.F. Acute nicotinamide deficiency in pigs. Vet. Rec. 1978, 103, 543–544. [Google Scholar] [CrossRef]
- Varma, L.P. Mental Symptoms in Pellagra and Nicotinic Acid Deficiency. Ind. Med. Gaz. 1943, 78, 543–546. [Google Scholar]
- Mc, F.A. Dermatitis as a monosymptomatic manifestation of nicotinic acid deficiency. Glasgow Med. J. 1947, 28, 103–115. [Google Scholar]
- Valdes, V.A.; Bolo, P.O. Nicotinic acid deficiency without pellagra; radiological changes in colon due to deficiency. Prensa Med. Argent. 1953, 40, 2622–2628. [Google Scholar] [PubMed]
- Zheng, M.; Cai, J.; Liu, Z.; Shu, S.; Wang, Y.; Tang, C.; Dong, Z. Nicotinamide reduces renal interstitial fibrosis by suppressing tubular injury and inflammation. J. Cell. Mol. Med. 2019, 23, 3995–4004. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Schilling, E.; Grahnert, A.; Kolling, V.; Dorow, J.; Ceglarek, U.; Sack, U.; Hauschildt, S. Nicotinamide: A vitamin able to shift macrophage differentiation toward macrophages with restricted inflammatory features. Innate Immun. 2015, 21, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Hiromatsu, Y.; Sato, M.; Tanaka, K.; Ishisaka, N.; Kamachi, J.; Nonaka, K. Inhibitory effects of nicotinamide on intercellular adhesion molecule-1 expression on cultured human thyroid cells. Immunology 1993, 80, 330–332. [Google Scholar] [PubMed]
- Hiromatsu, Y.; Sato, M.; Yamada, K.; Nonaka, K. Inhibitory effects of nicotinamide on recombinant human interferon-gamma-induced intercellular adhesion molecule-1 (ICAM-1) and HLA-DR antigen expression on cultured human endothelial cells. Immunol. Lett. 1992, 31, 35–39. [Google Scholar] [CrossRef]
- Biedron, R.; Ciszek, M.; Tokarczyk, M.; Bobek, M.; Kurnyta, M.; Slominska, E.M.; Smolenski, R.T.; Marcinkiewicz, J. 1-Methylnicotinamide and nicotinamide: Two related anti-inflammatory agents that differentially affect the functions of activated macrophages. Arch. Immunol. Ther. Exp. 2008, 56, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuzawa, M.; Satoh, J.; Muto, G.; Muto, Y.; Nishimura, S.; Miyaguchi, S.; Qiang, X.L.; Toyota, T. Inhibitory effect of nicotinamide on in vitro and in vivo production of tumor necrosis factor-alpha. Immunol. Lett. 1997, 59, 7–11. [Google Scholar] [CrossRef]
- Ungerstedt, J.S.; Blomback, M.; Soderstrom, T. Nicotinamide is a potent inhibitor of proinflammatory cytokines. Clin. Exp. Immunol. 2003, 131, 48–52. [Google Scholar] [CrossRef]
- Ungerstedt, J.S.; Heimersson, K.; Soderstrom, T.; Hansson, M. Nicotinamide inhibits endotoxin-induced monocyte tissue factor expression. J. Thromb. Haemost. 2003, 1, 2554–2560. [Google Scholar] [CrossRef] [Green Version]
- Grange, P.A.; Raingeaud, J.; Calvez, V.; Dupin, N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J. Dermatol. Sci. 2009, 56, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Permezel, M. The anti-inflammatory and antioxidative effects of nicotinamide, a vitamin B(3) derivative, are elicited by FoxO3 in human gestational tissues: Implications for preterm birth. J. Nutr. Biochem. 2011, 22, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Yanez, M.; Jhanji, M.; Murphy, K.; Gower, R.M.; Sajish, M.; Jabbarzadeh, E. Nicotinamide Augments the Anti-Inflammatory Properties of Resveratrol through PARP1 Activation. Sci. Rep. 2019, 9, 10219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstadter, J.; Kroller-Schon, S.; Munzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogata, S.; Takeuchi, M.; Teradaira, S.; Yamamoto, N.; Iwata, K.; Okumura, K.; Taguchi, H. Radical scavenging activities of niacin-related compounds. Biosci. Biotechnol. Biochem. 2002, 66, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Kamat, J.P.; Devasagayam, T.P. Nicotinamide (vitamin B3) as an effective antioxidant against oxidative damage in rat brain mitochondria. Redox Rep. 1999, 4, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.; Zheng, D.; Zhang, L.; Ni, R.; Wang, G.; Fan, G.C.; Lu, Z.; Peng, T. Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis. Free Radic. Biol. Med. 2018, 123, 125–137. [Google Scholar] [CrossRef]
- Mendez-Lara, K.A.; Santos, D.; Farre, N.; Ruiz-Nogales, S.; Leanez, S.; Sanchez-Quesada, J.L.; Zapico, E.; Lerma, E.; Escola-Gil, J.C.; Blanco-Vaca, F.; et al. Administration of CORM-2 inhibits diabetic neuropathy but does not reduce dyslipidemia in diabetic mice. PLoS ONE 2018, 13, e0204841. [Google Scholar] [CrossRef] [Green Version]
- Ribas, V.; Palomer, X.; Roglans, N.; Rotllan, N.; Fievet, C.; Tailleux, A.; Julve, J.; Laguna, J.C.; Blanco-Vaca, F.; Escola-Gil, J.C. Paradoxical exacerbation of combined hyperlipidemia in human apolipoprotein A-II transgenic mice treated with fenofibrate. Biochim. Biophys. Acta 2005, 1737, 130–137. [Google Scholar] [CrossRef]
- Escola-Gil, J.C.; Lee-Rueckert, M.; Santos, D.; Cedo, L.; Blanco-Vaca, F.; Julve, J. Quantification of In Vitro Macrophage Cholesterol Efflux and In Vivo Macrophage-Specific Reverse Cholesterol Transport. Methods Mol. Biol. 2015, 1339, 211–233. [Google Scholar]
- Maor, I.; Hayek, T.; Coleman, R.; Aviram, M. Plasma LDL oxidation leads to its aggregation in the atherosclerotic apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhang, S.; Blander, G.; Tse, J.G.; Krieger, M.; Guarente, L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell 2007, 28, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Turley, S.D.; Lobaccaro, J.A.; Medina, J.; Li, L.; Lustig, K.; Shan, B.; Heyman, R.A.; Dietschy, J.M.; Mangelsdorf, D.J. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 2000, 289, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldan, A.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Elenkov, I.J.; Chrousos, G.P. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. N. Y. Acad. Sci. 2002, 966, 290–303. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Lara, K.A.; Santos, D.; Farre, N.; Nan, M.N.; Pallares, V.; Perez-Perez, A.; Alonso, N.; Escola-Gil, J.C.; Blanco-Vaca, F.; Julve, J. Vitamin B3 impairs reverse cholesterol transport in Apolipoprotein E-deficient mice. Clin. Investig. Arterioscler. 2019, 31, 251–260. [Google Scholar]
- Hwang, E.S.; Song, S.B. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells. Cell. Mol. Life Sci. 2017, 74, 3347–3362. [Google Scholar] [CrossRef]
- Liu, D.; Gharavi, R.; Pitta, M.; Gleichmann, M.; Mattson, M.P. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromol. Med. 2009, 11, 28–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee-Rueckert, M.; Escola-Gil, J.C.; Kovanen, P.T. HDL functionality in reverse cholesterol transport—Challenges in translating data emerging from mouse models to human disease. Biochim. Biophys. Acta 2016, 1861, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Whitney, K.D.; Watson, M.A.; Goodwin, B.; Galardi, C.M.; Maglich, J.M.; Wilson, J.G.; Willson, T.M.; Collins, J.L.; Kliewer, S.A. Liver X receptor (LXR) regulation of the LXRalpha gene in human macrophages. J. Biol. Chem. 2001, 276, 43509–43515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laffitte, B.A.; Joseph, S.B.; Walczak, R.; Pei, L.; Wilpitz, D.C.; Collins, J.L.; Tontonoz, P. Autoregulation of the human liver X receptor alpha promoter. Mol. Cell. Biol. 2001, 21, 7558–7568. [Google Scholar] [CrossRef] [Green Version]
- Bradley, M.N.; Hong, C.; Chen, M.; Joseph, S.B.; Wilpitz, D.C.; Wang, X.; Lusis, A.J.; Collins, A.; Hseuh, W.A.; Collins, J.L.; et al. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J. Clin. Investig. 2007, 117, 2337–2346. [Google Scholar] [CrossRef]
- Ma, A.Z.; Song, Z.Y.; Zhang, Q. Cholesterol efflux is LXRalpha isoform-dependent in human macrophages. BMC Cardiovasc. Disord. 2014, 14, 80. [Google Scholar] [CrossRef] [Green Version]
- Cedo, L.; Metso, J.; Santos, D.; Garcia-Leon, A.; Plana, N.; Sabate-Soler, S.; Rotllan, N.; Rivas-Urbina, A.; Mendez-Lara, K.A.; Tondo, M.; et al. LDL Receptor Regulates the Reverse Transport of Macrophage-Derived Unesterified Cholesterol via Concerted Action of the HDL-LDL Axis: Insight From Mouse Models. Circ. Res. 2020, 127, 778–792. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Kitamoto, S.; Lian, Q.; Boisvert, W.A. Interleukin-10 facilitates both cholesterol uptake and efflux in macrophages. J. Biol. Chem. 2009, 284, 32950–32958. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.F.; Yang, X.F.; Cheng, B.; Mei, C.L.; Li, Q.X.; Xiao, H.; Zeng, Q.T.; Liao, Y.H.; Liu, K. Protective effect of Astragalus polysaccharides on ATP binding cassette transporter A1 in THP-1 derived foam cells exposed to tumor necrosis factor-alpha. Phytother. Res. 2010, 24, 393–398. [Google Scholar] [CrossRef]
- Reiss, A.B.; Carsons, S.E.; Anwar, K.; Rao, S.; Edelman, S.D.; Zhang, H.; Fernandez, P.; Cronstein, B.N.; Chan, E.S. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum. 2008, 58, 3675–3683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, X.R.; Cao, D.L.; Hu, Y.W.; Li, X.X.; Liu, X.H.; Xiao, J.; Liao, D.F.; Xiang, J.; Tang, C.K. IFN-gamma down-regulates ABCA1 expression by inhibiting LXRalpha in a JAK/STAT signaling pathway-dependent manner. Atherosclerosis 2009, 203, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lee, J.Y.; Timmins, J.M.; Brown, J.M.; Boudyguina, E.; Mulya, A.; Gebre, A.K.; Willingham, M.C.; Hiltbold, E.M.; Mishra, N.; et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 2008, 283, 22930–22941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateuszuk, L.; Jasztal, A.; Maslak, E.; Gasior-Glogowska, M.; Baranska, M.; Sitek, B.; Kostogrys, R.; Zakrzewska, A.; Kij, A.; Walczak, M.; et al. Antiatherosclerotic Effects of 1-Methylnicotinamide in Apolipoprotein E/Low-Density Lipoprotein Receptor-Deficient Mice: A Comparison with Nicotinic Acid. J. Pharmacol. Exp. Ther. 2016, 356, 514–524. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Wang, M.; Song, J.; Liu, Y.; Chen, H.; Mu, D.; Xia, M. N-methylnicotinamide protects against endothelial dysfunction and attenuates atherogenesis in apolipoprotein E-deficient mice. Mol. Nutr. Food Res. 2016, 60, 1625–1636. [Google Scholar] [CrossRef]



| Parameters | Untreated | NAM LD | NAM HD | p |
|---|---|---|---|---|
| Gross parameters | ||||
| Body weight (g) | 28.7 (27.0; 29.9) | 27.4 (23.3; 28.1) | 24.6 (22.3; 26.0) * | <0.05 |
| Liver weight (g) | 1.3 (1.2; 1.4) | 1.3 (1.2;1.4) | 1.2 (1.1;1.4) | 0.20 |
| Diet intake (g/day) | 2.5 (2.4; 2.8) | 2.7 (2.6; 2.8) | 2.4 (2.1; 2.5) † | <0.05 |
| Water intake (g/day) | 3.9 (3.2; 5.1) | 4.6 (4.4; 4.8) | 4.6 (3.8; 5.4) | 0.20 |
| Calculated dose of NAM (g/kg/day) | - | 0.5 (0.4; 0.7) | 1.9 (1.6; 2.2) † | <0.05 |
| Plasma biochemistry | ||||
| NAM (µM) | 4.0 (3.3; 5.5) | 193.5 (90.5, 248.5) | 580.0 (526.0; 605.0) * | <0.05 |
| me-NAM (relative values) (×10−3) a | 0.15 (0.13; 0.16) | 0.28 (0.19; 0.31) | 0.85 (0.45; 0.89) * | <0.05 |
| Glucose (mM) | 12.3 (10.6; 15.3) | 11.1 (9.4; 11.8) | 10.9 (8.4; 12.9) | 0.17 |
| Insulin (μg/L) | 0.7 (0.6; 0.8) | n. d. | 0.7 (0.6; 0.7) | 0.32 |
| Triglycerides (mM) | 0.5 (0.4; 1.0) | 1.8 (1.3; 2.5) * | 1.5 (1.1; 2.3) * | <0.05 |
| Total cholesterol (mM) | 43.3 (33.7; 47.4) | 53.0 (44.2; 55.2) | 77.6 (71.6, 82.3) * † | <0.05 |
| Non-HDL cholesterol (mM) | 43.1 (33.7; 47.0) | 52.6 (43.8; 54.9) | 77.3 (71.2, 82.0) * † | <0.05 |
| HDL cholesterol (mM) | 0.2 (0.1; 0.4) | 0.4 (0.3; 0.4) | 0.4 (0.1; 0.5) | 0.45 |
| AST (U/L) | 61 (24;128) | 25 (19; 39) | 63 (27; 117) | 0.33 |
| ALT (U/L) | 16 (7; 48) | 5 (3; 6) * | 6 (2; 11) * | <0.05 |
| Creatinine (mM) | 0.02 (0.01; 0.03) | 0.01 (0.01; 0.02) | 0.01 (0.01; 0.02) | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Lara, K.A.; Letelier, N.; Farré, N.; Diarte-Añazco, E.M.G.; Nieto-Nicolau, N.; Rodríguez-Millán, E.; Santos, D.; Pallarès, V.; Escolà-Gil, J.C.; Vázquez del Olmo, T.; et al. Nicotinamide Prevents Apolipoprotein B-Containing Lipoprotein Oxidation, Inflammation and Atherosclerosis in Apolipoprotein E-Deficient Mice. Antioxidants 2020, 9, 1162. https://doi.org/10.3390/antiox9111162
Méndez-Lara KA, Letelier N, Farré N, Diarte-Añazco EMG, Nieto-Nicolau N, Rodríguez-Millán E, Santos D, Pallarès V, Escolà-Gil JC, Vázquez del Olmo T, et al. Nicotinamide Prevents Apolipoprotein B-Containing Lipoprotein Oxidation, Inflammation and Atherosclerosis in Apolipoprotein E-Deficient Mice. Antioxidants. 2020; 9(11):1162. https://doi.org/10.3390/antiox9111162
Chicago/Turabian StyleMéndez-Lara, Karen Alejandra, Nicole Letelier, Núria Farré, Elena M. G. Diarte-Añazco, Núria Nieto-Nicolau, Elisabeth Rodríguez-Millán, David Santos, Victor Pallarès, Joan Carles Escolà-Gil, Tania Vázquez del Olmo, and et al. 2020. "Nicotinamide Prevents Apolipoprotein B-Containing Lipoprotein Oxidation, Inflammation and Atherosclerosis in Apolipoprotein E-Deficient Mice" Antioxidants 9, no. 11: 1162. https://doi.org/10.3390/antiox9111162