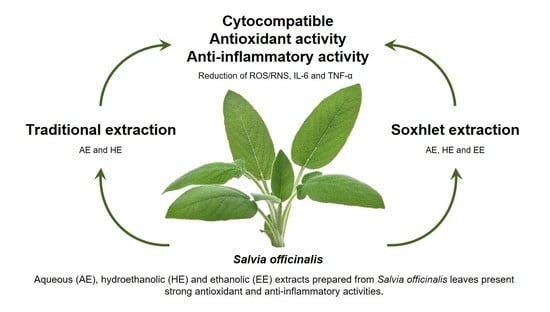
Antioxidant and Anti-Inflammatory Activities of Cytocompatible Salvia officinalis Extracts: A Comparison between Traditional and Soxhlet Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bioactive Compounds Extraction
2.3. Determination of the TPC
2.4. Determination of the TFC
2.5. Chromatographic Analyses
2.6. Preparation of S. officinalis Extracts Solutions and IC50 Calculation for Antioxidant Activity Assays
2.6.1. DPPH● Radical Scavenging Activity
2.6.2. ABTS●+ Radical Scavenging Activity
2.6.3. Antioxidant Activity against ROO●
2.6.4. Antioxidant Activity against ●NO
2.6.5. Antioxidant Activity against O2●−
2.6.6. RP Capacity
2.7. Preparation of S. officinalis Extracts Solution for Biological Studies
2.8. Cytotoxicity Evaluation
2.8.1. Cell Culture and Seeding
2.8.2. Metabolic Activity
2.8.3. DNA Quantification
2.8.4. Total Protein Content
2.8.5. Cell Morphology
2.9. Proinflammatory Activity Evaluation
Metabolic Activity
2.10. Anti-Inflammatory Activity Evaluation
IL-6 and TNF-α Quantification
2.11. Statistical Analysis
3. Results
3.1. Extraction Yield
3.2. TPC and TFC
3.3. TLC Analysis of S. officinalis Extracts
3.4. Antiradical Activity of S. officinalis Extracts against DPPH● and ABTS●+
3.5. Antioxidant Activity against ROO●
3.6. Antioxidant Activity against ●NO
3.7. Antioxidant Activity against O2●−
3.8. RP Scavenging Activity
3.9. Cytotoxicity of S. officinalis Extracts
3.9.1. L929 Cell Line
3.9.2. Non- and LPS-Stimulated Macrophages
3.10. Pro- and Anti-Inflammatory Activity of S. officinalis Extracts
3.10.1. Nonstimulated Macrophages
3.10.2. LPS-Stimulated Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, M.C.; Andriantsitohaina, R. Reactive nitrogen species: Molecular mechanisms and potential significance in health and disease. Antioxid. Redox Signal. 2009, 11, 669–702. [Google Scholar] [CrossRef] [PubMed]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.-C.; Gougerot-Pocidalo, M.-A.; Dang, P.M.-C. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Iles, K.E.; Forman, H.J. Macrophage signaling and respiratory burst. Immunol. Res. 2002, 26, 95–105. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448. [Google Scholar] [CrossRef]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signalling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, S14–S22. [Google Scholar] [CrossRef]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou-Raya, S.; Abou-Raya, A.; Naim, A.; Abuelkheir, H. Chronic inflammatory autoimmune disorders and atherosclerosis. Ann. N. Y. Acad. Sci. 2007, 1107, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; He, Y.; Chen, S.; Qi, S.; Shen, J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharmacol. Res. 2020, 158, 104877. [Google Scholar] [CrossRef] [PubMed]
- Steinmeyer, J. Pharmacological basis for the therapy of pain and inflammation with nonsteroidal anti-inflammatory drugs. Arthritis Res. 2000, 2, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Misery, L.; Naeyaert, S.; Taylor, P.C. Biological Therapies in Immune-Mediated Inflammatory Diseases: Can Biosimilars Reduce Access Inequities? Front. Pharmacol. 2019, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Harirforoosh, S.; Jamali, F. Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin. Drug Saf. 2009, 8, 669–681. [Google Scholar] [CrossRef]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef]
- Patrono, C. Cardiovascular Effects of Nonsteroidal Anti-inflammatory Drugs. Curr. Cardiol. Rep. 2016, 18, 25. [Google Scholar] [CrossRef]
- Chamoun-Emanuelli, A.M.; Bryan, L.K.; Cohen, N.D.; Tetrault, T.L.; Szule, J.A.; Barhoumi, R.; Whitfield-Cargile, C.M. NSAIDs disrupt intestinal homeostasis by suppressing macroautophagy in intestinal epithelial cells. Sci. Rep. 2019, 9, 14534. [Google Scholar] [CrossRef] [Green Version]
- Buchman, A.L. Side Effects of Corticosteroid Therapy. J. Clin. Gastroenterol. 2001, 33, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Swart, J.F.; de Roock, S.; Wulffraat, N.M. What are the immunological consequences of long-term use of biological therapies for juvenile idiopathic arthritis? Arthritis Res. Ther. 2013, 15, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivaji, U.N.; Sharratt, C.L.; Thomas, T.; Smith, S.C.L.; Iacucci, M.; Moran, G.W.; Ghosh, S.; Bhala, N. Review article: Managing the adverse events caused by anti-TNF therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 49, 664–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO (World Health Organization). The World Traditional Medicines Situation, in Traditional Medicines: Global Situation, Issues and Challenges, 3rd ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, N.R.; Akerele, O.; Bingel, A.S.; Soejarto, D.D.; Guo, Z. Medicinal plants in therapy. Bull. World Health Organ. 1985, 63, 965–981. [Google Scholar] [CrossRef] [Green Version]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Gasparrini, M.; Giampieri, F.; Forbes-Hernandez, T.Y.; Afrin, S.; Cianciosi, D.; Reboredo-Rodriguez, P.; Varela-Lopez, A.; Zhang, J.; Quiles, J.L.; Mezzetti, B.; et al. Strawberry extracts efficiently counteract inflammatory stress induced by the endotoxin lipopolysaccharide in Human Dermal Fibroblast. Food Chem. Toxicol. 2018, 114, 128–140. [Google Scholar] [CrossRef]
- Kuo, X.; Herr, D.R.; Ong, W.-Y. Anti-inflammatory and Cytoprotective Effect of Clinacanthus nutans Leaf But Not Stem Extracts on 7-Ketocholesterol Induced Brain Endothelial Cell Injury. Neuromol. Med. 2020. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Brown, O.P. The Complete Herbalist: Newcastle Classic Series; Newcastle Pub.: Jesery, UK, 1897; ISBN 9780878771844. [Google Scholar]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological notes about ancient uses of medicinal plants in Tras-os-Montes (northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhofer-Ressler, M.M.; Fricke, K.; Pignitter, M.; Walker, J.M.; Walker, J.; Rychlik, M.; Somoza, V. Identification of 1,8-cineole, borneol, camphor, and thujone as anti-inflammatory compounds in a Salvia officinalis L. infusion using human gingival fibroblasts. J. Agric. Food Chem. 2013, 61, 3451–3459. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, S.; Zhu, T.; Xue, G.; Xu, D.; Wang, W.; Wang, X.; Luo, J.; Kong, L. Anti-inflammatory norabietane diterpenoids from the leaves of Salvia officinalis L. J. Funct. Foods 2019, 54, 154–163. [Google Scholar] [CrossRef]
- Lima, C.F.; Andrade, P.B.; Seabra, R.M.; Fernandes-Ferreira, M.; Pereira-Wilson, C. The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats. J. Ethnopharmacol. 2005, 97, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.R.A.; Kanazawa, L.K.S.; das Neves, T.L.M.; da Silva, C.F.; Horst, H.; Pizzolatti, M.G.; Santos, A.R.S.; Baggio, C.H.; Werner, M.F. de P. Antinociceptive and anti-inflammatory potential of extract and isolated compounds from the leaves of Salvia officinalis in mice. J. Ethnopharmacol. 2012, 139, 519–526. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 10993-5:2009—Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.P.; Troganis, A.; Boskou, D. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J. Agric. Food Chem. 2002, 50, 5294–5299. [Google Scholar] [CrossRef]
- Cidade, H.; Rocha, V.; Palmeira, A.; Marques, C.; Tiritan, M.E.; Ferreira, H.; Lobo, J.S.; Almeida, I.F.; Sousa, M.E.; Pinto, M. In silico and in vitro antioxidant and cytotoxicity evaluation of oxygenated xanthone derivatives. Arab. J. Chem. 2020, 13, 17–26. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Duenas, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of phenolic compounds and antioxidant properties of Glycyrrhiza glabra L. rhizomes and roots. RSC Adv. 2015, 5, 26991–26997. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lúcio, M.; Nunes, C.; Gaspar, D.; Ferreira, H.; Lima, J.L.F.C.; Reis, S. Antioxidant Activity of Vitamin E and Trolox: Understanding of the Factors that Govern Lipid Peroxidation Studies In Vitro. Food Biophys. 2009, 4, 312–320. [Google Scholar] [CrossRef]
- Dorta, E.; Fuentes-Lemus, E.; Aspée, A.; Atala, E.; Speisky, H.; Bridi, R.; Lissi, E.; López-Alarcón, C. The ORAC (oxygen radical absorbance capacity) index does not reflect the capacity of antioxidants to trap peroxyl radicals. RSC Adv. 2015, 5, 39899–39902. [Google Scholar] [CrossRef]
- Pardau, M.D.; Pereira, A.S.P.; Apostolides, Z.; Serem, J.C.; Bester, M.J. Antioxidant and anti-inflammatory properties of Ilex guayusa tea preparations: A comparison to Camellia sinensis teas. Food Funct. 2017, 8, 4601–4610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, E.; Costa, D.; Toste, S.A.; Lima, J.L.F.C.; Reis, S. In vitro scavenging activity for reactive oxygen and nitrogen species by nonsteroidal anti-inflammatory indole, pyrrole, and oxazole derivative drugs. Free Radic. Biol. Med. 2004, 37, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Feelisch, M.; Noack, E.A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur. J. Pharmacol. 1987, 139, 19–30. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Analytical Chemistry of Nitric Oxide. Annu. Rev. Anal. Chem. 2009, 2, 409–433. [Google Scholar] [CrossRef] [Green Version]
- Vieira, S.; Franco, A.R.; Fernandes, E.M.; Amorim, S.; Ferreira, H.; Pires, R.A.; Reis, R.L.; Martins, A.; Neves, N.M. Fish sarcoplasmic proteins as a high value marine material for wound dressing applications. Colloids Surf. B Biointerfaces 2018, 167, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.C.; Cunha, C.; Carvalho, A.; Ferreira, H.; Neves, N.M. Interleukin-6 Neutralization by Antibodies Immobilized at the Surface of Polymeric Nanoparticles as a Therapeutic Strategy for Arthritic Diseases. ACS Appl. Mater. Interfaces 2018, 10, 13839–13850. [Google Scholar] [CrossRef]
- Bachiega, T.F.; Sforcin, J.M. Lemongrass and citral effect on cytokines production by murine macrophages. J. Ethnopharmacol. 2011, 137, 909–913. [Google Scholar] [CrossRef] [Green Version]
- Brahmi, F.; Nury, T.; Debbabi, M.; Hadj-Ahmed, S.; Zarrouk, A.; Prost, M.; Madani, K.; Boulekbache-Makhlouf, L.; Lizard, G. Evaluation of Antioxidant, Anti-Inflammatory and Cytoprotective Properties of Ethanolic Mint Extracts from Algeria on 7-Ketocholesterol-Treated Murine RAW 264.7 Macrophages. Antioxidants 2018, 7, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Farhat, M.; Jordán, M.J.; Chaouech-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Variations in Essential Oil, Phenolic Compounds, and Antioxidant Activity of Tunisian Cultivated Salvia officinalis L. J. Agric. Food Chem. 2009, 57, 10349–10356. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Cuvelier, M.E.; Berset, C.; Richard, H. Antioxidant Constituents in Sage (Salvia officinalis). J. Agric. Food Chem. 1994, 42, 665–669. [Google Scholar] [CrossRef]
- Sallam, A.; Mira, A.; Ashour, A.; Shimizu, K. Acetylcholine esterase inhibitors and melanin synthesis inhibitors from Salvia officinalis. Phytomedicine 2016, 23, 1005–1011. [Google Scholar] [CrossRef]
- Maksimovic, S.; Kesic, Z.; Lukic, I.; Milovanovic, S.; Ristic, M.; Skala, D. Supercritical fluid extraction of curry flowers, sage leaves, and their mixture. J. Supercrit. Fluids 2013, 84, 1–12. [Google Scholar] [CrossRef]
- Radulescu, V.; Chiliment, S.; Oprea, E. Capillary gas chromatography–mass spectrometry of volatile and semi-volatile compounds of Salvia officinalis. J. Chromatogr. A 2004, 1027, 121–126. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry 2000, 55, 263–267. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Tada, M.; Hara, T.; Hara, C.; Chiba, K. A quinone methide from Salvia officinalis. Phytochemistry 1997, 45, 1475–1477. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Albano, S.M.; Miguel, M.G. Biological activities of extracts of plants grown in Portugal. Ind. Crops Prod. 2011, 33, 338–343. [Google Scholar] [CrossRef]
- Farhat, M.B.; Landoulsi, A.; Chaouch-Hamada, R.; Sotomayor, J.A.; Jordán, M.J. Characterization and quantification of phenolic compounds and antioxidant properties of Salvia species growing in different habitats. Ind. Crops Prod. 2013, 49, 904–914. [Google Scholar] [CrossRef]
- Nanni, V.; Canuti, L.; Gismondi, A.; Canini, A. Hydroalcoholic extract of Spartium junceum L. flowers inhibits growth and melanogenesis in B16-F10 cells by inducing senescence. Phytomedicine 2018, 46, 1–10. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Mokwena, L.M.; Opara, U.L. Effect of extraction method on chemical, volatile composition and antioxidant properties of pomegranate juice. S. Afr. J. Bot. 2016, 103, 135–144. [Google Scholar] [CrossRef]
- Leelarungrayub, N.; Rattanapanone, V.; Chanarat, N.; Gebicki, J.M. Quantitative evaluation of the antioxidant properties of garlic and shallot preparations. Nutrition 2006, 22, 266–274. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: Peppermint, melissa, and sage. J. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef]
- Jesionek, W.; Majer-Dziedzic, B.; Choma, I.M. Separation, Identification, and Investigation of Antioxidant Ability of Plant Extract Components Using TLC, LC–MS, and TLC–DPPH•. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1147–1153. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Rangarajan, M.; Shao, Y.; LaVoie, E.J.; Huang, T.-C.; Ho, C.-T. Antioxidative Phenolic Compounds from Sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Antioxidant activities of polyphenols from sage (Salvia officinalis). Food Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Atoui, A.K.; Mansouri, A.; Boskou, G.; Kefalas, P. Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- Kozics, K.; Klusová, V.; Srančíková, A.; Mučaji, P.; Slameňová, D.; Hunáková, Ľ.; Kusznierewicz, B.; Horváthová, E. Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 2013, 141, 2198–2206. [Google Scholar] [CrossRef]
- Pereira, O.R.; Catarino, M.D.; Afonso, A.F.; Silva, A.M.S.; Cardoso, S.M. Salvia elegans, Salvia greggii and Salvia officinalis Decoctions: Antioxidant Activities and Inhibition of Carbohydrate and Lipid Metabolic Enzymes. Molecules 2018, 23, 3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontush, A. Amyloid-β: An antioxidant that becomes a pro-oxidant and critically contributes to Alzheimer’s disease. Free Radic. Biol. Med. 2001, 31, 1120–1131. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ban, I.; Choi, Y.; Yu, S.; Youn, S.J.; Baik, M.-Y.; Lee, H.; Kim, W. Puffing of Turmeric (Curcuma longa L.) Enhances its Anti-Inflammatory Effects by Upregulating Macrophage Oxidative Phosphorylation. Antioxidants 2020, 9, 931. [Google Scholar] [CrossRef] [PubMed]
- Arranz, E.; Jaime, L.; Lopez de la Hazas, M.C.; Vicente, G.; Reglero, G.; Santoyo, S. Supercritical sage extracts as anti-inflammatory food ingredients. Ind. Crops Prod. 2014, 54, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Lu, Y.; Yang, F.; Li, S.; He, X.; Gao, Y.; Zhang, G.; Ren, E.; Wang, Y.; Kang, X. Rosmarinic acid exerts a neuroprotective effect on spinal cord injury by suppressing oxidative stress and inflammation via modulating the Nrf2/HO-1 and TLR4/NF-κB pathways. Toxicol. Appl. Pharmacol. 2020, 397, 115014. [Google Scholar] [CrossRef]
- Xia, G.; Wang, X.; Sun, H.; Qin, Y.; Fu, M. Carnosic acid (CA) attenuates collagen-induced arthritis in db/db mice via inflammation suppression by regulating ROS-dependent p38 pathway. Free Radic. Biol. Med. 2017, 108, 418–432. [Google Scholar] [CrossRef]
- Shi, W.; Xu, G.; Zhan, X.; Gao, Y.; Wang, Z.; Fu, S.; Qin, N.; Hou, X.; Ai, Y.; Wang, C.; et al. Carnosol inhibits inflammasome activation by directly targeting HSP90 to treat inflammasome-mediated diseases. Cell Death Dis. 2020, 11, 252. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, S.F.; Ferreira, H.; Neves, N.M. Antioxidant and Anti-Inflammatory Activities of Cytocompatible Salvia officinalis Extracts: A Comparison between Traditional and Soxhlet Extraction. Antioxidants 2020, 9, 1157. https://doi.org/10.3390/antiox9111157
Vieira SF, Ferreira H, Neves NM. Antioxidant and Anti-Inflammatory Activities of Cytocompatible Salvia officinalis Extracts: A Comparison between Traditional and Soxhlet Extraction. Antioxidants. 2020; 9(11):1157. https://doi.org/10.3390/antiox9111157
Chicago/Turabian StyleVieira, Sara F., Helena Ferreira, and Nuno M. Neves. 2020. "Antioxidant and Anti-Inflammatory Activities of Cytocompatible Salvia officinalis Extracts: A Comparison between Traditional and Soxhlet Extraction" Antioxidants 9, no. 11: 1157. https://doi.org/10.3390/antiox9111157
APA StyleVieira, S. F., Ferreira, H., & Neves, N. M. (2020). Antioxidant and Anti-Inflammatory Activities of Cytocompatible Salvia officinalis Extracts: A Comparison between Traditional and Soxhlet Extraction. Antioxidants, 9(11), 1157. https://doi.org/10.3390/antiox9111157