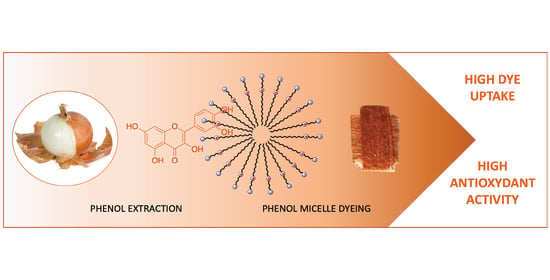
Use of a Zwitterionic Surfactant to Improve the Biofunctional Properties of Wool Dyed with an Onion (Allium cepa L.) Skin Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Photophysical Measurements
2.3. Scouring and Dyeing Procedure
2.4. Preparation of Artificial Sweat
2.5. Treatment of Textiles with Artificial Sweat
2.6. Determination of Total Phenol Content (TPC) by the Folin–Ciocalteu Method
2.7. Determination of the Total Antioxidant Capacity (TAC) by the FRAP Method
2.8. Determination of the Oxygen Radical Absorbance Capacity by the ORAC Method
2.9. Determination of the Radical Scavenging Capacity by the DPPH Method
2.10. Determination of the Radical Scavenging Capacity by the TEAC/ABTS Method
2.11. HPLC Analysis of Polyphenols in the Extracts
2.12. Analysis of Cellular Viability and Apoptosis of SB3-14 on RAW 264.7 Cells
3. Results and Discussion
3.1. Interaction of Quercetin with SB3-14 Surfactant
3.2. Effect of SB3-14 on Dyed Wool Properties
3.3. Quantitative Analysis of Phenolic Acids and Flavonols in the Extracts
3.4. Determination of SB3-14 Cytotoxicity on RAW 264.7 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grifoni, D.; Bacci, L.; Di Lonardo, S.; Pinelli, P.; Scardigli, A.; Camilli, F.; Sabatini, F.; Zipoli, G.; Romani, R. UV protective properties of cotton and flax fabrics dyed with multifunctional plant extracts. Dye. Pigm. 2014, 105, 89–96. [Google Scholar] [CrossRef]
- Islama, S.; Wanib, S.A.; Mohammada, F. Imparting functionality viz color, antioxidant and antibacterial properties to develop multifunctional wool with Tectona grandis leaves extract using reflectance. Int. J. Biol. Macromol. 2018, 109, 907–913. [Google Scholar] [CrossRef]
- Chen, C.; Chang, W.Y. Antimicrobial activity of cotton fabric pretreated with microwave plasma and dyed with onion skin and onion pulp extractions. Indian J. Fibre Text. Res. 2007, 32, 122–125. [Google Scholar]
- Upadhayay, H.; Jahan, S.; Upreti, M. Cosmetotextiles: Emerging Trend in Technical Textiles. IOSR J. Polym. Text. Eng. 2016, 3, 8–14. [Google Scholar]
- Saharan, M.; Saini, H.K.; Kaur, M. Cosmetotextiles: A novel technique of developing wearable skin care. Asian J. Home Sci. 2017, 12, 289–295. [Google Scholar]
- Lis, M.J.; Martí, M.; Coderch, L.; Alonso, C.; Bezerra, F.M.; Immich, A.P.; Tornero, J.A. Biofunctional Textiles. In Advances in Textile Engineering, 1st ed.; Open Access e Books: Las Vegas, NV, USA, 2007; pp. 1–28. [Google Scholar]
- Shahid, M.; Islam, S.U.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, R.-C. Natural Flavonoid-Functionalized Silk Fiber Presenting Antibacterial, Antioxidant, and UV Protection Performance. ACS Sustain. Chem. Eng. 2017, 5, 10518–10526. [Google Scholar] [CrossRef]
- Hong, K.H. Phenol compounds treated cotton and wool fabrics for developing multi-functional clothing materials. Fibers Polym. 2015, 16, 565–571. [Google Scholar] [CrossRef]
- Ghaheh, F.S.; Mortazavi, S.M.; Alihosseini, F.; Fassihi, A.; Nateri, A.S.; Abedi, D. Assessment of antibacterial activity of wool fabrics dyed with natural dyes. J. Clean. Prod. 2014, 72, 139–145. [Google Scholar] [CrossRef]
- Pucciarini, L.; Ianni, F.; Petesse, V.; Pellati, F.; Brighenti, V.; Volpi, C.; Gargaro, M.; Natalini, B.; Clementi, C.; Sardella, R. Onion (Allium cepa L.) skin: A rich resource of biomolecules for the sustainable production of colored biofunctional textiles. Molecules 2019, 24, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojana, B.P.; Marika, S. Microencapsulation technology and applications in added-value functional textiles. Phys. Sci. Rev. 2016, 1, 1–27. [Google Scholar] [CrossRef]
- Chandravanshi, S.; Upadhyay, S.K. Natural dye—surfactant interactions: Thermodynamic and surface parameters. Color. Technol. 2012, 128, 300–305. [Google Scholar] [CrossRef]
- Chandravanshi, S.; Upadhyay, S.K. Interaction of natural dye (Allium caepa) with ionic surfactants. J. Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Baliarsingh, S.; Jena, J.; Das, T.; Das, N.B. Role of cationic and anionic surfactants in textile dyeing with natural dyes extracted from waste plant materials and their potential antimicrobial properties. Ind. Crops Prod. 2013, 50, 618–624. [Google Scholar] [CrossRef]
- Myers, D. Surfactant Science and Technology, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Horiuchi, S.; Aoyama, Y. Systematic Lactose-Functionalization of Amphiphilic Octaamine Macrocycle as a Gene Carrier. Optimization of the Charge, Size, Toxicity, and Receptor Factors for Hepatocyte Targeting. J. Control. Release 2006, 116, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Zhang, Y.; Zhou, J.; Zou, A.; Yu, D.; Wu, Y.; Li, J.; Li, H. Synthesis and Characterization of Low-Toxic Amphiphilic Chitosan Derivatives and Their Application as Micelle Carrier for Antitumor. Drug. Int. J. Pharm. 2010, 394, 162–173. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Vyas, S.P. Non-Ionic Surfactant Based Vesicles (Niosomes) in Drug Delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Tiecco, M.; Roscini, L.; Corte, L.; Colabella, C.; Germani, R.; Cardinali, G. Ionic Conductivity as a Tool to Study Biocidal Activity of Sulfobetaine Micelles against Saccharomyces cerevisiae Model Cells. Langmuir 2016, 32, 1101–1110. [Google Scholar] [CrossRef]
- Clementi, C.; Cesaretti, A.; Carlotti, B.; Elisei, F. The Role of pH in Modulating the Electronic State Properties of Minocycline Drug and Its Inclusion within Micellar Carriers. J. Phys. Chem. A 2016, 120, 4994–5005. [Google Scholar] [CrossRef]
- Costas-Costas, U.; Bravo-Diaz, C.; Gonzalez-Romero, E. Sodium Dodecyl Sulfate Micellar Effects on the Reaction between Arenediazonium Ions and Ascorbic Acid Derivatives. Langmuir 2003, 19, 5197–5203. [Google Scholar] [CrossRef]
- Gentili, P.L.; Clementi, C.; Romani, A. Ultraviolet–Visible Absorption and Luminescence Properties of Quinacridone-Barium Sulfate Solid Mixtures. Appl. Spectrosc. 2010, 64, 923–929. [Google Scholar] [CrossRef]
- Bacci, M.; Baronti, S.; Casini, A.; Lotti, F.; Picollo, M.; Casazza, O. Non-destructive spectroscopic investigations on paintings using optical fibers. Mat. Res. Soc. Symp. Proc. 1992, 267, 265–283. [Google Scholar] [CrossRef]
- Lisanti, A.; Formica, V.; Ianni, F.; Albertini, B.; Marinozzi, M.; Sardella, R.; Natalini, B. Anyioxidant activity of phenolic extracts from different cultivars of Italian onion (Allium Cepa) and relative humane immune cell proliferative induction. Pharm. Biol. 2016, 54, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Gramza-Michalowska, A.; Korczak, J. Oxygen radical absorbance capacity of selected food products. Acta Sci. Pol. Technol. Aliment. 2013, 12, 175–180. [Google Scholar]
- Álvarez-Diduk, R.; Ramírez-Silva, M.T.; Galano, A.; Merkoçi, A. Deprotonation Mechanism and Acidity Constants in Aqueous Solution of Flavonols: A Combined Experimental and Theoretical Study. J. Phys. Chem. B 2013, 117, 12347–12359. [Google Scholar] [CrossRef]
- Alva-Mezzetti, A.; Protti, S.; Lapouge, C.; Cornard, J.P. Protic equilibria as the key factor of quercetin emission in solution. Relevance to biochemical and analytical studies. Phys. Chem. Chem. Phys. 2011, 13, 6858–6864. [Google Scholar] [CrossRef]
- Chakraborty, J.N. Fundamentals and Practices in Colouration of Textiles, 1st ed.; Woodhead Publishing India PVt. Ltd.: New Deli, India, 2010. [Google Scholar]
- Cesaretti, A.; Carlotti, B.; Gentili, P.L.; Clementi, C.; Germani, R.; Elisei, F. Spectroscopic Investigation of the pH Controlled Inclusion of Doxycycline and Oxytetracycline Antibiotics in Cationic Micelles and their Magnesium Driven Release. J. Phys. Chem. B 2014, 118, 8601–8613. [Google Scholar] [CrossRef]
- Liu, W.; Guo, R. Interaction between flavonoid, quercetin and surfactant aggregates with different charges. J. Colloid Interface Sci. 2006, 302, 625–632. [Google Scholar] [CrossRef]
- Singh, O.; Kaur, R.; Kumar-Mahajan, R. Flavonoid-surfactant interactions: A detailed physicochemical study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 170, 77–88. [Google Scholar] [CrossRef]
- Alva-Ensastegui, J.C.; Palomar-Pardavé, M.; Romero-Romo, M.; Ramìrez-Silva, M.T. Quercetin spectrofluorometric quantification in aqueous media using different surfactants as fluorescence prompoters. RSC Adv. 2018, 8, 10980–10986. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.-R.; In, M.Y.; Yuan, Y.; Lee, J.-J.; Kim, S. In situ Spectroelectrochemical Study of Quercetin Oxidation and complexation with Metal Ions in Acidic Solutions. Bull. Korean Chem. Soc. 2007, 28, 889–892. [Google Scholar]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT) and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef] [PubMed]







| Sample | L* | a* | b* |
|---|---|---|---|
| T1 | 55.44 | 13.48 | 25.53 |
| T2 | 50.24 | 15.26 | 23.39 |
| Sample | Type of Assay | ||||
|---|---|---|---|---|---|
| FOLIN–CIOCALTEU | FRAP | DPPH | ABTS | ORAC | |
| mg Eq GA/g Textile | mg Eq TROLOX/g Textile | ||||
| T1 | 17.29 ± 0.67 | 5.98 ± 0.50 | 2.14 ± 1.16 | 4.17 ± 2.15 | 9.38 ± 1.72 |
| T2 | 21.82 ± 2.77 | 7.97 ± 1.70 | 4.62 ± 1.66 | 4.34 ± 0.24 | 11.41 ± 1.54 |
| Sample | Protocatechuic acid and Derivatives (µg PAE/mL) | Quercetin and Derivatives (µg QE/mL) |
|---|---|---|
| ET1 | 100.0 ± 11.0 | 26.2 ± 3.1 |
| ET1-post | 91.8 ± 2.7 | 22.0 ± 0.1 |
| ET2 | 109.1 ± 4.3 | 32.4 ± 3.5 |
| ET2-post | 26.2 ± 3.1 | 15.7 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puri, C.; Pucciarini, L.; Tiecco, M.; Brighenti, V.; Volpi, C.; Gargaro, M.; Germani, R.; Pellati, F.; Sardella, R.; Clementi, C. Use of a Zwitterionic Surfactant to Improve the Biofunctional Properties of Wool Dyed with an Onion (Allium cepa L.) Skin Extract. Antioxidants 2020, 9, 1055. https://doi.org/10.3390/antiox9111055
Puri C, Pucciarini L, Tiecco M, Brighenti V, Volpi C, Gargaro M, Germani R, Pellati F, Sardella R, Clementi C. Use of a Zwitterionic Surfactant to Improve the Biofunctional Properties of Wool Dyed with an Onion (Allium cepa L.) Skin Extract. Antioxidants. 2020; 9(11):1055. https://doi.org/10.3390/antiox9111055
Chicago/Turabian StylePuri, Chiara, Lucia Pucciarini, Matteo Tiecco, Virginia Brighenti, Claudia Volpi, Marco Gargaro, Raimondo Germani, Federica Pellati, Roccaldo Sardella, and Catia Clementi. 2020. "Use of a Zwitterionic Surfactant to Improve the Biofunctional Properties of Wool Dyed with an Onion (Allium cepa L.) Skin Extract" Antioxidants 9, no. 11: 1055. https://doi.org/10.3390/antiox9111055