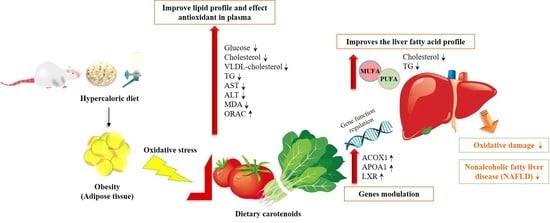
4.1. Carotenoid Intake and its Action on Steatosis Biomarkers
Several authors have pointed out that dietary fat, cholesterol and fructose are the main drivers of the development and progression of obesity and steatosis [
22,
23]. In the present study, an atherogenic (21% fat and 1.25% cholesterol) and high-fructose (20%) diet was used for the induction of hepatic steatosis for eight weeks. The disease was confirmed by histopathological analysis of the liver, increased plasma triglycerides and the activity of liver enzymes in plasma, which were outside the range considered normal [
32]. Taking into consideration the carotenoid intake and the average weight of the rats (0.5 kg), the ingested dose of carotenoids was 8 and 16 mg/kg/day in LC12.75 and HC25.5, respectively, and these values are within those that have been considered safe in different toxicity studies. Specifically, the Panel of the European Food Safety Authority (EFSA), after evaluating a study carried out with rats, has considered a maximum dose with no observed adverse effect level (NOAEL) of 500 mg/kg/day for lycopene, 538 mg/kg/day for lutein and 150 mg/kg/day for zeaxanthin, since, although they accumulate in the liver, the presence of pigments without the existence of histopathological alterations is not relevant from a toxicological point of view [
33,
34].
Firstly, supplementation of the feed with tomato and spinach concentrates was sufficient to significantly reduce the bodyweight of the rats by 5% and 8%, respectively. In overweight people at metabolic risk, a 5% reduction in body weight has been shown to reduce the incidence of type 2 diabetes by 58% [
35]; hence, a relatively moderate weight loss is capable of causing improvements in metabolic control. This fact directly affects the treatment of NAFLD, since overweight and diabetes play a fundamental role in the appearance of the disease [
36,
37]. This effect could be associated with lower caloric intake, but other factors in the experimental diets may also influence weight control, such as the higher consumption of TDF. Parallel to the changes observed in body weight, supplementation with the mixture of spinach and tomato improved the lipid profile of the plasma, notably reducing the levels of glucose, total cholesterol, VLDL, triglycerides, proteins, ALT and AST compared to the control group. This lipid-lowering effect of carotenoids has been described in other previous studies in obese animals with NAFLD [
38,
39] and is due to different mechanisms. Thus, lycopene has been shown to have a hypocholesterolemic effect, as it is capable of inhibiting the cholesterol synthesis limiting enzyme 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) [
40,
41], although it is also capable of modulating the expression of genes involved in lipid metabolism, mainly due to the modulation of genes involved in the signaling pathways of PPARs [
10,
11,
12,
14]. The lipid-lowering and hypocholesterolemic effects are directly related to the accumulation of lycopene after ingestion, since this was the majority carotenoid in the experimental diets and the only carotenoid found at the plasma level, with concentrations ranging from 13 to 15 ng/mL, as a function of lycopene concentration in the diets. These values coincide with the lycopene levels reported by Luvizotto et al. [
42], who found a mean value of 12.67 ng of lycopene/mL after consumption of 3.5 mg of this carotenoid by Wistar rats. The ORAC value represents an adequate biochemical parameter to assess the general status of antioxidants [
25,
43], and it increased after the consumption of a mixture of spinach and tomato in the diet, since, parallel to the increase in lycopene, ORAC in plasma was increased.
Although carotenoids other than lycopene were not detected in the plasma, the carotenoids present in the experimental feeds were accumulated in the liver, with the HC25.5 group showing the highest content. Therefore, the different carotenoids, namely lutein, zeaxanthin, phytoene, β-carotene and lycopene, were absorbed and accumulated in the liver to later be incorporated into the lipoproteins for release into the circulation [
44]. In the liver, these micronutrients exert a clear antioxidant effect, acting effectively against free radical species, which results in less lipoperoxidation and a significant decrease in MDA content. This effect was clearly observed in the LC12.75 and HC25.5 groups, since MDA levels were significantly lower than in the control group, and, in the latter, a greater accumulation of MDA as a result of excessive lipid peroxidation was observed [
45]. This fact is also reflected in other oxidative stress biomarkers such as MDA content in plasma and urine. These data coincide with those reported by other researchers, who observed that some carotenoids such as lycopene, β-carotene and lutein decrease MDA in plasma and liver of rats and guinea pigs with steatosis and NASH induced by a hypercholesterolemic diet or by carbon tetrachloride (CCL
4) [
15,
39,
46]. Thus, carotenoids play an important role in defense against oxidative stress by preventing or delaying oxidation through the neutralization of free radicals by sequestering singlet oxygen [
47]. Carotenoids are considered beneficial for the prevention and treatment of NAFLD due to the role they play in reducing oxidative damage [
48] in the diet-mediated recuperation of steatosis.
Although carotenoids were absorbed and accumulated in the liver, a fecal analysis determined that 100% of the ingested carotenoids were not absorbed and were excreted in the feces, as has been described by other authors [
49,
50]. Although the maximum absorption of these bioactive compounds is always sought, there are many factors that can affect their intestinal absorption, thus it must be taken into account that a proportion of them will reach the colon, where they can affect the intestinal microbiota or even act as antioxidants for colon cells [
51].
Recent studies in animal models indicate that the accumulation of fat and cholesterol in the hepatocyte are key factors for the activation of the inflammatory pathways involved in the transition from NAFLD to NASH and to hepatocellular carcinoma in animal models [
52]. The intake of carotenoid-rich diets (groups LC12.75 and HC25.5) decreased the concentrations of total cholesterol and liver triglycerides, as has been found in other investigations with the supplementation of lutein, lycopene, astaxanthin and β-carotene [
14,
16,
53,
54]. The livers of the rats from the LC12.75 and HC25.5 groups, in addition to having a lower steatosis score than that of the CD group, showed less cholesterol accumulation, possibly due to its mobilization by plasma HDL [
52,
53]. This could explain the decrease in the concentration of cholesterol in the liver. Furthermore, as previously mentioned, other mechanisms that could contribute to the reduction of cholesterol in the liver are the hypocholesterolemic effect of lycopene (majority carotenoid accumulated in the liver), which inhibits the activity of HMGCR; the regulation of gene expression involved in lipid metabolism [
11,
40,
41]; and an increase in the bile acid secretion [
55]. However, this last mechanism was not evaluated in this study since we did not analyze the content of bile acids in the feces. It is worth mentioning that, even though a complete recovery from steatosis was not observed in the microscopic study, the differences in the size of the vacuoles associated with the intake of carotenoids were evident. Likewise, the intake of dietary carotenoids showed a positive effect by effectively decreasing the levels of total cholesterol and liver triglycerides, decreasing lipid accumulation and helping to restore liver function.
MUFAs and PUFAs increased while SAFAs and the ratio of n-6/n-3 decreased after the intake of carotenoid-rich diets. MUFAs and PUFAs exert a beneficial effect on the liver, modulating the activity of liver cells during liver fibrosis [
56]. This increase in MUFAs can be beneficial for liver health, since different authors have shown that MUFAs can improve insulin resistance, increase the release of triglycerides in the liver, decrease lipolytic flow of peripheral adipose tissue [
57,
58] and inhibit kinases related to oxidative stress and apoptosis [
59] in human and animal models with NAFLD. Moreover, the increase in the proportion of PUFAs suggests that the accumulation of hepatic carotenoids stimulates the conversion of fatty acids into long-chain unsaturated products, as mentioned in the previous study with the supplementation of spinach in the diet [
14]. The production of ALA (C18:3n-3 α), DGLA (C20:3n-3), EPA (C20: 5n-3) and DHA (C22:6n-3) was increased in the LC12.75 and HC25.5 groups. In general, n-3 AGPs have been shown to have effects against obesity, steatosis and inflammation [
60], as well as glucose and lipid metabolism [
61]. These effects could be due to the stimulation of fat catabolism and the inhibition of de novo fatty acid synthesis [
57]. In our study, the increase in n-3 fatty acids was significantly correlated with the accumulation of carotenoids and the decrease of lipids in the liver. Furthermore, the decrease in the n-6/n-3 ratio has an effect on the reduction of hepatic triglyceride storage [
57], as observed in the present study, contributing carotenoids in the diet-mediated recuperation of steatosis.
4.2. Bioactive Components of Spinach and Tomato Modulate Gene Expression Related to Steatosis
In this study, we observed that the ingestion of a diet rich in carotenoids after the induction of steatosis and during its dietetic treatment leads to changes in the expression of genes related to fatty liver disease. The main carotenoids of the diets, lycopene and lutein, are transported into the hepatocyte by the transmembrane transporter and by fatty acid-binding protein into the cytosol, where carotenoids act as activators of different nuclear receptors, which are involved in the transcriptional regulation of several pathways of lipid metabolism [
62] (
Figure 3).
The overexpression of the
ACOX1 gene, as well as the synthesis of peroxisomal ACOX1 protein, could indicate a healthier status to maintain the peroxisome activity of β-oxidation. Thus, these findings suggest that the accumulation of carotenoids in the liver favors the peroxisomal lipid oxidation by improving the breakdown of medium and very long-chain fatty acids. Consequently, the carotenoids could aid in the recuperation of the homeostasis of liver lipids, as has been reported in other studies [
54,
63]. In fact, we observed a significant decrease in cholesterol, TGA and SAFA in the liver, decreasing the lipid peroxidation and protecting the liver against inflammation and fibrosis. It is recognized that this protein plays an important role in the NAFLD, as ACOX1-deficient mice have shown spontaneous steatosis and steatohepatitis, as well as the spontaneous development of hepatocellular carcinoma (HCC) [
64]. Similarly, it has been observed that patients with pseudoneonatal adrenoleukodystrophy (a disease characterized by ACOX1 deficiency) show hepatomegaly due to impaired peroxisomal fatty acid β-oxidation of very long-chain fatty acids [
65].
ACADL, which encodes a dehydrogenase enzyme that catalyzes the initial step in each cycle of mitochondrial fatty acid β-oxidation [
66], was also overexpressed in the group LC12.75; this effect was also described after the consumption of tomato juice in rats with steatosis [
11].
Furthermore, the genes involved in cholesterol transport and metabolism increased their activity, overexpressing
APOA1,
CNBP,
NR1H2 and
PPARD in the HC25.5 group. The
APOA1 gene encodes apolipoprotein A-1, which is the main protein of HDL in plasma, which defines its size and shape, solubilizes its lipid component and helps to reverse cholesterol transport [
67]. It also acts as a cofactor of lecithin cholesterol acyltransferase (LCAT) for the formation of most cholesterol esters in plasma, promoting the release of cholesterol from the tissues to the liver for its subsequent excretion [
68]. Recent studies indicate that metabolic alterations in the body, including changes in the levels and quality of APOA1, may favor the appearance and progression of some types of cancer, such as HCC, showing that the development of hepatocellular carcinoma is associated with lower levels of apolipoprotein A-1 [
69,
70,
71]. The findings of this study indicate that a greater expression of the APOA1 protein is proportional to the carotenoids ingested in the diet. The high consumption of carotenoids could promote atheroprotective properties, since the overexpression of
APOA1 could improve the reverse cholesterol transport, decreasing the cholesterol content in the liver and improving the hallmarks of the steatosis and then the progression of NAFLD [
72].
CNBP was also overexpressed in the liver after the consumption of a diet with high carotenoid content. This gene is expressed in a wide variety of tissues, is regulated by sterols and plays an important role in the control of sterol-mediated transcription [
73]. Similarly, rats from the HC25.5 group showed an expression of the
LXRB gene (
NR1H2) and the synthesis of its protein (LXRβ). These lipid-activated transcription factors heterodimerize with the Retinoid X Receptor (RXR) to control cholesterol and fatty acid homeostasis by regulating the expression of multiple enzymes, transporters and modulators involved in this process and also by modulating inflammatory and immune pathways. Hence, LXRs have been proposed as effective therapeutic agents against NAFLD [
74]. Recently, Becares et al. [
75] reported that LXRs phosphorylation acts as a molecular sensor in response to nutritional challenges, thus promoting a diet-induced transcriptome that modulates metabolic and inflammatory responses in NAFLD progression. Therefore, accumulation of carotenoids could play an important role in the phosphorylation of these transcription factors, leading to a decrease in lipids accumulation on the hepatocyte and of oxidative stress biomarkers.
The ingestion of spinach and tomato modulated lipid metabolism in the LC12.75 group, overexpressing the
ACSM3 gene, which encodes a protein that participates in the synthesis of medium-chain fatty acids, and showing an increase in lipid metabolism. As mentioned in a previous study, the activity of this encoded protein facilitates the oxidation of medium-chain fatty acids (C4–C11); for this reason, rats fed carotenoids had a lower SAFA/TFA ratio at the liver level [
14], as can be seen in
Table 4. The
HNF4A gene encodes the nuclear transcription factor protein that binds to DNA, controlling the expression of several liver genes (nuclear hepatocyte factor 1 alpha), also regulating the genes involved in the metabolism of cholesterol, fatty acids and glucose [
76,
77].
In this study, we confirmed that the consumption of carotenoids from spinach and tomato facilitates the recuperation of steatosis, and that the accumulation of carotenoids in the liver also seems to have a protective effect on the diet-mediated recuperation of steatosis. The ameliorative effect is mediated through the modulation of genes involved in lipid metabolism, especially by improving the β-oxidation of peroxisomal fatty acids through ACOX1, improving the homeostasis of cholesterol and fatty acids through LXRB and promoting the release of cholesterol from tissues to the liver for subsequent excretion thanks to APOA1. Therefore, our findings provide a new perspective on the nutritional value of dietary carotenoids from tomato and spinach in the treatment of NAFLD.
The present study describes the influence of the synergistic effect of two foods rich in carotenoids (neoaxanthin, violaxanthin, lutein, zeaxanthin, phytoene, α-carotene, β-carotene and lycopene) with two different concentrations of these bioactive compounds in the diet. This research was carried out with experimental animals (Sprague-Dawley rats) in which hepatic steatosis was previously induced. Our results provide additional evidence of the synergistic and potential effect of dietary carotenoids on liver health and dietary treatment of steatosis, suggesting that the carotenoids provided by the spinach and tomato are effective in reducing the accumulation of lipids in the liver, oxidative stress and inflammation of hepatocytes and increasing the body’s antioxidant activity, modulating the intracellular signaling pathways of the β-oxidation of fatty acids and the metabolism of lipids and cholesterol at the genomic and proteomic level.
A limitation of the present study is that, as it was carried out with animals, it cannot be completely extrapolated to humans, so a clinical study is needed in both healthy people without diseases and those with steatosis problems due to excessive consumption of fats and carbohydrates. Such a study would aim to corroborate the protective function of dietary carotenoids as well as evaluate whether there could be an effective dose, considering the bioavailability of dietary carotenoids, for the prevention of NAFLD.