Dendropanax morbifera Leaf Extracts Improved Alcohol Liver Injury in Association with Changes in the Gut Microbiota of Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction
2.2. Total Polyphenol and Flavonoid Contents
2.3. Antioxidant Activity
2.4. HPLC Analysis of Phenolic Acid and Flavonoid Components of D. morbifera Extracts
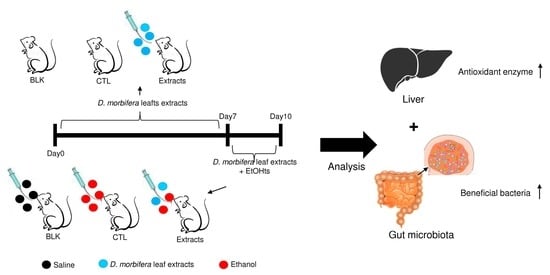
2.5. Animal Experiments
2.6. Serum Biochemical Analysis
2.7. Antioxidant Enzyme Activity
2.8. Hepatic Histopathological Observation
2.9. Analysis of Gut Microbiota
2.10. Statistical Analysis
3. Results
3.1. Total Polyphenol and Flavonoid Contents
3.2. Antioxidant Activity
3.3. Flavonoid and Phenolic Acid Analyses of D. morbifera Leaf Extract
3.4. Effects of DML Extracts on Suppression of Liver Damage
3.5. Reduction of Ethanol and Acetaldehyde in Blood with DML Extract Intake
3.6. Effects of DML Extracts on Antioxidant Enzymes in Liver Tissues
3.7. Microbiota Shifted by DML Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sookoian, S.; Pirola, C.J. Systems biology elucidates common pathogenic mechanisms between nonalcoholic and alcoholic-fatty liver disease. PLoS ONE 2013, 8, e58895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratna, A.; Mandrekar, P. Alcohol and cancer: Mechanisms and therapies. Biomolecules 2017, 7, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druesne-Pecollo, N.; Tehard, B.; Mallet, Y.; Gerber, M.; Norat, T.; Hercberg, S.; Latino-Martel, P. Alcohol and genetic polymorphisms: Effect on risk of alcohol-related cancer. Lancet Oncol. 2009, 10, 173–180. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Hashimoto, N.; Fukushima, M. Relationships among alcoholic liver disease, antioxidants, and antioxidant enzymes. World J. Gastroenterol. 2016, 22, 37–49. [Google Scholar] [CrossRef]
- Alonso, V.R.; Guarner, F. Linking the gut microbiota to human health. Brit. J. Nutr. 2013, 109, S21–S26. [Google Scholar] [CrossRef] [Green Version]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.C.; Finlay, B.B. The commensal microbiota drives immune homeostasis. Front. Immunol. 2012, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Wieland, A.; Frank, D.N.; Harnke, B.; Bambha, K. Systematic review: Microbial dysbiosis and nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2015, 42, 1051–1063. [Google Scholar] [CrossRef]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Vassallo, G.; Mirijello, A.; Ferrulli, A.; Antonelli, M.; Landolfi, R.; Gasbarrini, A.; Addolorato, G. Alcohol and gut microbiota-the possible role of gut microbiota modulation in the treatment of alcoholic liver disease. Aliment. Pharmacol. Ther. 2015, 41, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.B.; Tian, K.; Huang, L.L.; He, C.W.; Jiang, Y.; Wang, Y.T.; Wan, J.B. Herbal medicines for the prevention of alcoholic liver disease: A review. J. Ethnopharmacol. 2012, 144, 457–465. [Google Scholar] [CrossRef]
- Seeff, L.B.; Bonkovsky, H.L.; Navarro, V.J.; Wang, G.Q. Herbal products and the liver: A review of adverse effects and mechanisms. Gastroenterology 2015, 148, 517–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, G.S.; Lim, D.P.; Kim, S.M.; Yoo, E.S.; Kim, S.H.; Kim, C.H.; Woo, J.S.; Kim, H.J.; Jung, J.Y. Anti-inflammatory effects of Dendropanax morbifera in lipopolysaccharide-stimulated raw264.7 macrophages and in an animal model of atopic dermatitis. Mol. Med. Rep. 2019, 19, 2087–2096. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.Y.; Chung, T.H.; Hwang, I.K. Dendropanax morbifera leveille extract ameliorates memory impairments and inflammatory responses in the hippocampus of streptozotocin-induced type 1 diabetic rats. Mol. Cell. Toxicol. 2016, 12, 429–436. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, M.O.; Lee, H.; Kim, Y.; Kim, E.; Kim, J.S. Evaluation of anti-oxidant and anti-cancer properties of Dendropanax morbifera leveille. Food Chem. 2013, 141, 1947–1955. [Google Scholar] [CrossRef]
- Park, J.U.; Kang, B.Y.; Kim, Y.R. Ethyl acetate fraction from Dendropanax morbifera leaves increases t cell growth by upregulating nf-at-mediated il-2 secretion. Am. J. Chin. Med. 2018, 46, 453–467. [Google Scholar] [CrossRef]
- Eom, T.; Kim, K.C.; Kim, J.S. Dendropanax morbifera leaf polyphenolic compounds: Optimal extraction using the response surface method and their protective effects against alcohol-induced liver damage. Antioxidants 2020, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Hyun, T.K.; Kim, H.C.; Ko, Y.J.; Kim, J.S. Antioxidant, alpha-glucosidase inhibitory and anti-inflammatory effects of aerial parts extract from korean crowberry (Empetrum nigrum var. Japonicum). Saudi J. Biol. Sci. 2016, 23, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Bae, D.; Kim, J.; Lee, S.Y.; Choi, E.J.; Jung, M.A.; Jeong, C.S.; Na, J.R.; Kim, J.J.; Kim, S. Hepatoprotective effects of aqueous extracts from leaves of Dendropanax morbifera leveille against alcohol-induced hepatotoxicity in rats and in vitro anti-oxidant effects. Food Sci. Biotechnol. 2015, 24, 1495–1503. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [PubMed]
- Aebi, H. Catalase invitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Koneru, M.; Sahu, B.D.; Gudem, S.; Kuncha, M.; Ravuri, H.G.; Kumar, J.M.; Kilari, E.K.; Sistla, R. Polydatin alleviates alcohol-induced acute liver injury in mice: Relevance of matrix metalloproteinases (mmps) and hepatic antioxidants. Phytomedicine 2017, 27, 23–32. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The silva ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. Vsearch: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Cole, J.R.; Chai, B.; Farris, R.J.; Wang, Q.; Kulam-Syed-Mohideen, A.; McGarrell, D.M.; Bandela, A.; Cardenas, E.; Garrity, G.M.; Tiedje, J.M. The ribosomal database project (RDP-II): Introducing myrdp space and quality controlled public data. Nucleic Acids Res. 2007, 35, D169–D172. [Google Scholar] [CrossRef] [Green Version]
- Westcott, S.L.; Schloss, P.D. Opticlust, an improved method for assigning amplicon-based sequence data to operational taxonomic units. mSphere 2017, 2, e00073-17. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. SIGMOBILE Mob. Comput. Commun. Rev. 2001, 5, 3–55. [Google Scholar] [CrossRef]
- Beals, E.W. Bray-curtis ordination: An effective strategy for analysis of multivariate ecological data. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 1984; pp. 1–55. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 1–5. [Google Scholar] [CrossRef]
- Fernandes, A.D.; Reid, J.N.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the analysis of high-throughput sequencing datasets: Characterizing rna-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obernier, J.A.; Bouldin, T.W.; Crews, F.T. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol. Clin. Exp. Res. 2002, 26, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.M. Emerging role of circadian clock disruption in alcohol-induced liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G364–G373. [Google Scholar] [CrossRef]
- Dunn, W.; Shah, V.H. Pathogenesis of alcoholic liver disease. Clin. Liver Dis. 2016, 20, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Li, Y.; Zhang, Y.J.; Zhou, Y.; Li, S.; Li, H.B. Natural products for the prevention and treatment of hangover and alcohol use disorder. Molecules 2016, 21, 64. [Google Scholar] [CrossRef]
- Halbwirth, H. The creation and physiological relevance of divergent hydroxylation patterns in the flavonoid pathway. Int. J. Mol. Sci. 2010, 11, 595–621. [Google Scholar] [CrossRef]
- Eom, T.K.; Senevirathne, M.; Kim, S.K. Synthesis of phenolic acid conjugated chitooligosaccharides and evaluation of their antioxidant activity. Environ. Toxicol. Pharmacol. 2012, 34, 519–527. [Google Scholar] [CrossRef]
- Desideri, E.; Ciccarone, F.; Ciriolo, M.R. Targeting glutathione metabolism: Partner in crime in anticancer therapy. Nutrients 2019, 11, 1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Jiang, Y.X.; Zhang, W.W.; Wang, J.B.; Wang, R.L.; Wang, L.F.; Wei, S.Z.; Wen, J.X.; Li, H.T.; Zhao, Y.L. Natural products for the prevention and treatment of cholestasis: A review. Phytother. Res. 2020, 34, 1291–1309. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. The beneficial role of rutin, a naturally occurring flavonoid in health promotion and disease prevention: A systematic review and update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Academic Press: Cambridge, MA, USA, 2019; pp. 457–479. [Google Scholar] [CrossRef]
- Li, L.; Su, C.P.; Chen, X.Y.; Wang, Q.; Jiao, W.C.; Luo, H.; Tang, J.Y.; Wang, W.; Li, S.; Guo, S.Z. Chlorogenic acids in cardiovascular disease: A review of dietary consumption, pharmacology, and pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef] [PubMed]
- Caslin, B.; Maguire, C.; Karmakar, A.; Mohler, K.; Wylie, D.; Melamed, E. Alcohol shifts gut microbial networks and ameliorates a murine model of neuroinflammation in a sex-specific pattern. Proc. Natl. Acad. Sci. USA 2019, 116, 25808–25815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Yin, P.; Fan, H.; Sun, L.; Liu, Y. Flaxseed oil ameliorates alcoholic liver disease via anti-inflammation and modulating gut microbiota in mice. Lipids Health Dis. 2017, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wang, L.; Yi, H.; Wu, X. Beneficial effects of lrp6-crispr on prevention of alcohol-related liver injury surpassed fecal microbiota transplant in a rat model. Gut Microbes 2020, 1–15. [Google Scholar] [CrossRef]
- Gu, Z.; Wu, Y.; Wang, Y.; Sun, H.; You, Y.; Piao, C.; Liu, J.; Wang, Y. Lactobacillus rhamnosus granules dose-dependently balance intestinal microbiome disorders and ameliorate chronic alcohol-induced liver injury. J. Med. Food 2020, 23, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Brandl, K.; Kumar, V.; Eckmann, L. Gut-liver axis at the frontier of host-microbial interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G413–G419. [Google Scholar] [CrossRef]
- Hyun, T.K.; Ko, Y.-J.; Kim, E.-H.; Chung, I.-M.; Kim, J.-S. Anti-inflammatory activity and phenolic composition of Dendropanax morbifera leaf extracts. Ind. Crop. Prod. 2015, 74, 263–270. [Google Scholar] [CrossRef]
- Greetham, H.L.; Gibson, G.R.; Giffard, C.; Hippe, H.; Merkhoffer, B.; Steiner, U.; Falsen, E.; Collins, M.D. Allobaculum stercoricanis gen. Nov., sp. Nov., isolated from canine feces. Anaerobe 2004, 10, 301–307. [Google Scholar] [CrossRef]
- Cresci, G.A.; Bush, K.; Nagy, L.E. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcohol. Clin. Exp. Res. 2014, 38, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.; Glueck, B.; McMullen, M.R.; Xin, W.; Allende, D.; Nagy, L.E. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J. Gastroenterol. Hepatol. 2017, 32, 1587–1597. [Google Scholar] [CrossRef]
- Ferrere, G.; Wrzosek, L.; Cailleux, F.; Turpin, W.; Puchois, V.; Spatz, M.; Ciocan, D.; Rainteau, D.; Humbert, L.; Hugot, C.; et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J. Hepatol. 2017, 66, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Takagaki, A.; Matsumoto, K.; Kato, Y.; Goto, K.; Benno, Y. Butyricimonas synergistica gen. Nov., sp. Nov. and Butyricimonas virosa sp. Nov., butyric acid-producing bacteria in the family ‘Porphyromonadaceae’ isolated from rat faeces. Int. J. Syst. Evol. Microbiol. 2009, 59, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Shkoporov, A.N.; Khokhlova, E.V.; Chaplin, A.V.; Kafarskaia, L.I.; Nikolin, A.A.; Polyakov, V.Y.; Shcherbakova, V.A.; Chernaia, Z.A.; Efimov, B.A. Coprobacter fastidiosus gen. nov., sp. nov., a novel member of the family Porphyromonadaceae isolated from infant faeces. Int. J. Syst. Evol. Microbiol. 2013, 63, 4181–4188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabari, L.; Gannoun, H.; Cayol, J.-L.; Hedi, A.; Sakamoto, M.; Falsen, E.; Ohkuma, M.; Hamdi, M.; Fauque, G.; Ollivier, B.; et al. Macellibacteroides fermentans gen. nov., sp. nov., a member of the family Porphyromonadaceae isolated from an upflow anaerobic filter treating abattoir wastewaters. Int. J. Syst. Evol. Microbiol. 2012, 62, 2522–2527. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Lin, Z.; Zeng, Y.; Lin, X.; Zhang, Y. Probiotic and glutamine treatments attenuate alcoholic liver disease in a rat model. Exp. Ther. Med. 2019, 18, 4733–4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M.J. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G168–G175. [Google Scholar] [CrossRef] [Green Version]
- Schoenhofen, I.C.; Vinogradov, E.; Whitfield, D.M.; Brisson, J.R.; Logan, S.M. The cmp-legionaminic acid pathway in campylobacter: Biosynthesis involving novel gdp-linked precursors. Glycobiology 2009, 19, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.R.; Briggs, W.; Duff, A.; Chasser, K.; Murugesan, R.; Pender, C.; Ramirez, S.; Valenzuela, L.; Bielke, L. Cecal microbiome composition and metabolic function in probiotic treated broilers. PLoS ONE 2020, 15, e0225921. [Google Scholar] [CrossRef]
- Fernández-Reina, A.; Urdiales, J.L.; Sánchez-Jiménez, F. What we know and what we need to know about aromatic and cationic biogenic amines in the gastrointestinal tract. Foods 2018, 7, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoek, J.B.; Cahill, A.; Pastorino, J.G. Alcohol and mitochondria: A dysfunctional relationship. Gastroenterology 2002, 122, 2049–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermudez, B.; Lopez, S.; Ortega, A.; Varela, L.M.; Pacheco, Y.M.; Abia, R.; Muriana, F.J. Oleic acid in olive oil: From a metabolic framework toward a clinical perspective. Curr. Pharm. Des. 2011, 17, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chassaing, B.; Zhang, L.; San Yeoh, B.; Xiao, X.; Kumar, M.; Baker, M.T.; Cai, J.; Walker, R.; Borkowski, K.; et al. Microbiota-dependent hepatic lipogenesis mediated by stearoyl coa desaturase 1 (scd1) promotes metabolic syndrome in tlr5-deficient mice. Cell Metab. 2015, 22, 983–996. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, J.; Dakshinamurti, K. Transcriptional regulation of the glucokinase gene by biotin in starved rats. J. Biol. Chem. 1991, 266, 10035–10038. [Google Scholar]
- Kim, J.Y.; Hwang, J.-Y.; Lee, D.Y.; Song, E.H.; Park, K.J.; Kim, G.H.; Jeong, E.A.; Lee, Y.J.; Go, M.J.; Kim, D.J.; et al. Chronic ethanol consumption inhibits glucokinase transcriptional activity by Atf3 and triggers metabolic syndrome in vivo. J. Biol. 2014, 289, 27065–27079. [Google Scholar] [CrossRef] [Green Version]
- Ke, J.; Li, Y.; Han, C.; He, R.; Lin, R.; Qian, W.; Hou, X. Fucose ameliorate intestinal inflammation through modulating the crosstalk between bile acids and gut microbiota in a chronic colitis murine model. Inflamm. Bowel Dis. 2020, 26, 863–873. [Google Scholar] [CrossRef]
- Tsuruya, A.; Kuwahara, A.; Saito, Y.; Yamaguchi, H.; Tsubo, T.; Suga, S.; Inai, M.; Aoki, Y.; Takahashi, S.; Tsutsumi, E.; et al. Ecophysiological consequences of alcoholism on human gut microbiota: Implications for ethanol-related pathogenesis of colon cancer. Sci. Rep. 2016, 6, 27923. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.W.; Ge, C.; Feng, G.X.; Li, Y.; Luo, D.; Dong, J.L.; Li, H.; Wang, H.; Cui, M.; Fan, S.J. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol. Lett. 2018, 287, 23–30. [Google Scholar] [CrossRef]
- Millman, J.; Okamoto, S.; Kimura, A.; Uema, T.; Higa, M.; Yonamine, M.; Namba, T.; Ogata, E.; Yamazaki, S.; Shimabukuro, M.; et al. Metabolically and immunologically beneficial impact of extra virgin olive and flaxseed oils on composition of gut microbiota in mice. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef] [Green Version]
- Houghton, D.; Stewart, C.J.; Stamp, C.; Nelson, A.; Aj Ami, N.J.; Petrosino, J.F.; Wipat, A.; Trenell, M.I.; Turnbull, D.M.; Greaves, L.C. Impact of age-related mitochondrial dysfunction and exercise on intestinal microbiota composition. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Flavonoids | DMLDE | DMLEE | DMSDE | DMSEE |
|---|---|---|---|---|
| Flavonoid glycoside (mg/g of extracts) | ||||
| Naringin | 0.848 ± 1.075 | 0.017 ± 0.014 | 0.116 ± 0.010 | 0.104 ± 0.184 |
| Narirutin | 0.176 ± 0.012 | 2.808 ± 0.015 | 0.329 ± 0.117 | 0.227 ± 0.173 |
| Neohesperidin | 0.185 ± 0.003 | 3.308 ± 0.009 | 0.351 ± 0.149 | 0.053 ± 0.037 |
| Hesperidin | 0.021 ± 0.002 | 1.256 ± 0.179 | 0.484 ± 0.003 | 0.374 ± 0.004 |
| Rutin | 15.723 ± 0.005 | 44.88 ± 0.436 | 0.122 ± 0.095 | 1.085 ± 0.059 |
| Flavonoid aglycone (mg/g of extracts) | ||||
| Apigenin | - | - | - | - |
| Hesperetin | - | - | - | - |
| Isorhaemnetin | 0.428 ± 0.001 | - | 0.385 ± 0.076 | 0.282 ± 0.096 |
| Kaempferol | - | - | - | - |
| Luteorin | - | - | - | - |
| Myricetin | 0.245 ± 0.001 | 0.306 ± 0.001 | 0.236 ± 0.004 | 0.203 ± 0.001 |
| Naringenin | 0.546 ± 0.007 | - | 0.406 ± 0.013 | 0.499 ± 0.034 |
| Quercetin | 0.149 ± 0.002 | 0.148 ± 0.001 | 0.020 ± 0.001 | 0.139 ± 0.001 |
| Rhaemnetin | - | - | - | - |
| Taxifolin | 4.369 ± 0.014 | 11.705 ± 0.029 | 1.253 ± 0.218 | 1.593 ± 0.023 |
| Polymethoxyflavone (mg/g of extracts) | ||||
| Nobiletin | 0.615 ± 0.012 | 0.574 ± 0.001 | - | - |
| Sinesetin | 0.542 ± 0.001 | 0.461 ± 0.073 | - | - |
| Tangeretin | 0.265 ± 0.002 | 0.579 ± 0.095 | - | - |
| Derivatives | DMLDE | DMLEE | DMSDE | DMSEE |
|---|---|---|---|---|
| Benzoic acid derivative (mg/g of extracts) | ||||
| Benzoic acid | 0.460 ± 0.002 | 1.081 ± 0.022 | 0.702 ± 0.073 | 0.638 ± 0.033 |
| p-Hydroxybenzoic acid | 0.294 ± 0.146 | 0.196 ± 0.040 | 0.167 ± 0.016 | 0.153 ± 0.003 |
| Protocatechuic acid | 0.663 ± 0.001 | 0.553 ± 0.001 | 0.466 ± 0.187 | 0.248 ± 0.001 |
| Vanillic acid | 0.611 ± 0.322 | 0.333 ± 0.053 | 0.843 ± 0.086 | 0.966 ± 0.045 |
| Syringic acid | 0.297 ± 0.002 | 0.371 ± 0.115 | 1.054 ± 0.534 | 0.454 ± 0.045 |
| Gallic acid | 0.653 ± 0.007 | 0.732 ± 0.003 | 0.597 ± 0.062 | 0.364 ± 0.315 |
| Cinnamic acid derivative (mg/g of extracts) | ||||
| Cinnamic acid | 0.164 ± 0.001 | 0.105 ± 0.001 | 0.161 ± 0.043 | 0.111 ± 0.001 |
| p-Coumaric acid | 0.679 ± 0.018 | 2.092 ± 0.247 | 0.654 ± 0.178 | 0.892 ± 0.078 |
| Caffeic acid | 13.850 ± 0.024 | 21.824 ± 1.356 | 11.072 ± 0.178 | 17.446 ± 0.286 |
| Ferullic acid | 0.440 ± 0.001 | 0.916 ± 0.003 | 0.452 ± 0.020 | 0.475 ± 0.006 |
| Sinapinic acid | 0.417 ± 0.001 | 0.359 ± 0.001 | 0.428 ± 0.016 | 0.563 ± 0.001 |
| Chlorogenic acid | 5.165 ± 0.004 | 2.945 ± 0.119 | 1.532 ± 0.637 | 0.811 ± 0.024 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, T.; Ko, G.; Kim, K.C.; Kim, J.-S.; Unno, T. Dendropanax morbifera Leaf Extracts Improved Alcohol Liver Injury in Association with Changes in the Gut Microbiota of Rats. Antioxidants 2020, 9, 911. https://doi.org/10.3390/antiox9100911
Eom T, Ko G, Kim KC, Kim J-S, Unno T. Dendropanax morbifera Leaf Extracts Improved Alcohol Liver Injury in Association with Changes in the Gut Microbiota of Rats. Antioxidants. 2020; 9(10):911. https://doi.org/10.3390/antiox9100911
Chicago/Turabian StyleEom, Taekil, Gwangpyo Ko, Kyeoung Cheol Kim, Ju-Sung Kim, and Tatsuya Unno. 2020. "Dendropanax morbifera Leaf Extracts Improved Alcohol Liver Injury in Association with Changes in the Gut Microbiota of Rats" Antioxidants 9, no. 10: 911. https://doi.org/10.3390/antiox9100911