An Overview of the Genus Cotoneaster (Rosaceae): Phytochemistry, Biological Activity, and Toxicology
Abstract
:1. Introduction
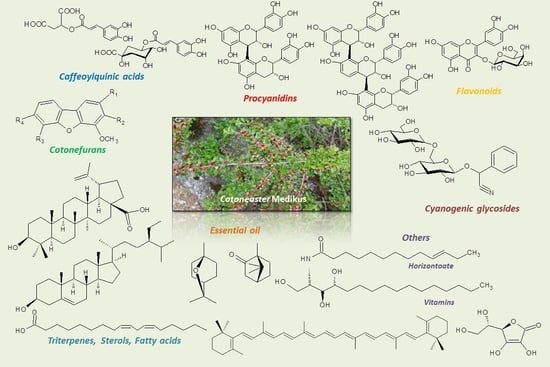
2. Phytochemical Composition of the Genus Cotoneaster
2.1. Flavonoids and Proanthocyanidins
2.2. Phenolic Acids
2.3. Phytoalexins
2.4. Cyanogenic Glycosides
2.5. Triterpenes, Sterols, and Fatty Acids
2.6. Essential Oil
2.7. Carbohydrates
2.8. Other Constituents
3. Biological Activities of the Genus Cotoneaster
3.1. Traditional Usages
3.2. Pharmacological Activities
3.2.1. Antioxidant Activity
3.2.2. Anti-Inflammatory Activity
3.2.3. Antimicrobial Activity
3.2.4. Antiparasitic Activity
3.2.5. Hepatoprotective, Anti-Diabetic, and Anti-Dyslipidaemic Effects
3.2.6. Enzyme Inhibitory Activity
3.2.7. Anti-Jaundice Activity
3.2.8. Cytotoxic Activity
4. Methodology
5. Conclusions
Funding
Conflicts of Interest
References
- Hummer, K.E.; Janick, J. Rosaceae: Taxonomy, economic importance, genomics. In Plant Genetics/Genomics: Genetics and Genomics of Rosaceae; Gardiner, S.E., Folta, K.M., Eds.; Springer: New York, NY, USA, 2009; Volume 6, pp. 1–17. [Google Scholar]
- Soundararajan, P.; Won, S.Y.; Kim, J.S. Insight on Rosaceae Family with genome sequencing and functional genomics perspective. Biomed. Res. Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dickoré, W.B.; Kasperek, G. Species of Cotoneaster (Rosaceae, Maloideae) indigenous to, naturalising or commonly cultivated in Central Europe. Willdenowia 2010, 40, 13–45. [Google Scholar] [CrossRef]
- Jerzak, E. Cotoneaster Species Cultivated in Poland; Officina Botanica: Krakow, Poland, 2007. [Google Scholar]
- Holzer, V.M.D.; Lower-Nedza, A.D.; Nandintsetseg, M.; Batkhuu, J.; Brantner, A.H. Antioxidant constituents of Cotoneaster melanocarpus Lodd. Antioxidants 2013, 2, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Ghiaee, A.; Naghibi, F.; Mosaddegh, M. Antiplasmodial activity and cytotoxicity of plants used in traditional medicine of Iran for the treatment of fever. Iran J. Pharm. Res. 2015, 14, 103–107. [Google Scholar] [PubMed]
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE 2014, 9, e113527. [Google Scholar] [CrossRef]
- Cooke, R.G.; Fletcher, R.A.H. Isoflavonoids. III. Constituents of Cotoneaster species. Aust. J. Chem. 1974, 27, 1377–1379. [Google Scholar] [CrossRef] [Green Version]
- Pashinina, L.T.; Chumbalov, T.K.; Sheichenko, V.I.; Shukenova, R.Z. Dimeric proanthocyanidins of Cotoneaster oligantha. Chem. Nat. Comp. 1978, 14, 166–172. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Pharmacological activities of Cotoneaster racemiflorus—A Review. Pharm. Chem. J. 2016, 3, 98–104. [Google Scholar]
- Swati, S.; Manjula, R.R.; Sowjanya, K.; Vennela, Y.; Tanuja, K. A phyto pharmacological review on Cotoneaster microphyllus species. J. Pharm. Sci. Res. 2018, 10, 2166–2168. [Google Scholar]
- Chumbalov, T.K.; Pashinina, L.T.; Shukenova, R.Z. Flavonoids of Cotoneaster oligantha. Chem. Nat. Comp. 1975, 11, 99. [Google Scholar] [CrossRef] [Green Version]
- Palme, E.; Bilia, A.R.; de Feo, V.; Morelli, I. Flavonoid glycosides from Cotoneaster thymaefolia. Phytochemistry 1994, 35, 1381–1382. [Google Scholar] [CrossRef]
- Palme, E.; Bilia, A.R.; Morelli, I. Flavonols and isoflavones from Cotoneaster simonsii. Phytochemistry 1996, 42, 903–905. [Google Scholar] [CrossRef]
- El-Mousallamy, A.M.; Hussein, S.A.; Merfort, I.; Nawwar, M.A. Unusual phenolic glycosides from Cotoneaster orbicularis. Phytochemistry 2000, 53, 699–704. [Google Scholar] [CrossRef]
- Sati, S.C.; Sati, M.; Sharma, A.; Joshi, M. Isolation and characterisation of phenolics from the roots of Cotoneaster acuminatus and determination of their antimicrobial activity. Int. J. Pharm. Pharm. Sci. 2010, 2, 58–60. [Google Scholar]
- Kicel, A.; Owczarek, A.; Kapusta, P.; Kolodziejczyk-Czepas, J.; Olszewska, M.A. Contribution of individual polyphenols to antioxidant activity of Cotoneaster bullatus and Cotoneaster zabelii leaves—Structural relationships, synergy effects and application for quality control. Antioxidants 2020, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Odontuya, G. Phytochemicals in leaves of Cotoneaster mongolica, their antioxidative, and acetylcholinesterase inhibitory activity. Mong. J. Chem. 2019, 20, 1–6. [Google Scholar]
- Kicel, A.; Michel, P.; Owczarek, A.; Marchelak, A.; Zyzelewicz, D.; Budryn, G.; Oracz, J.; Olszewska, M.A. Phenolic profile and antioxidant potential of leaves from selected Cotoneaster Medik. species. Molecules 2016, 21, 688. [Google Scholar] [CrossRef] [Green Version]
- Kicel, A.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Marchelak, A.; Sopinska, M.; Ciszewski, P.; Nowak, P.; Olszewska, M.A. Polyphenol-rich extracts from Cotoneaster leaves inhibit pro-inflammatory enzymes and protect human plasma components against oxidative stress in vitro. Molecules 2018, 23, 2472. [Google Scholar] [CrossRef] [Green Version]
- Kicel, A.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Rutkowska, M.; Wajs-Bonikowska, A.; Granica, S.; Nowak, P.; Olszewska, M.A. Multifunctional phytocompounds in Cotoneaster fruits: Phytochemical profiling, cellular safety, anti-inflammatory and antioxidant effects in chemical and human plasma models in vitro. Oxid. Med. Cell. Longev. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Kicel, A.; Owczarek, A.; Gralak, P.; Ciszewski, P.; Olszewska, M.A. Polyphenolic profile, antioxidant activity, and pro-inflammatory enzymes inhibition of leaves, flowers, bark and fruits of Cotoneaster integerrimus: A comparative study. Phytochem. Lett. 2019, 30, 349–355. [Google Scholar] [CrossRef]
- Ekin, H.N.; Gokbulut, A.; Aydin, Z.U.; Donmez, A.A. Insight into anticholinesterase and antioxidant potential of thirty-four Rosaceae samples and phenolic characterization of the active extracts by HPLC. Ind. Crops Prod. 2016, 91, 104–113. [Google Scholar] [CrossRef]
- Uysal, A.; Zengin, G.; Mollica, A.; Gunes, E.; Locatelli, M.; Yilmaz, T.; Aktumsek, A. Chemical and biological insights on Cotoneaster integerrimus: A new (-)-epicatechin source for food and medicinal applications. Phytomedicine 2016, 15, 979–988. [Google Scholar] [CrossRef]
- Les, F.; López, V.; Caprioli, G.; Iannarelli, R.; Fiorini, D.; Innocenti, M.; Bellumori, M.; Maggi, F. Chemical constituents, radical scavenging activity and enzyme inhibitory capacity of fruits from Cotoneaster pannosus Franch. Food Funct. 2017, 8, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Sokkar, N.M.; El-Gindi, O.; Ali, Z.Y.; Alfishawy, I.A. Phytoconstituents investigation, anti-diabetic and anti-dyslipidemic activities of Cotoneaster horizontalis Decne cultivated in Egypt. Life Sci. J. 2012, 9, 394–403. [Google Scholar]
- Khan, S.; Yasmeen, S.; Afza, N.; Malik, A.; Iqbal, L.; Lateef, M. Cotonoates A and B, new aromatic esters from Cotoneaster racemiflora. Zeitschrift für Naturforschung B 2008, 63, 1219–1222. [Google Scholar] [CrossRef]
- Khan, S.; Wang, Z.; Wang, R.; Zhang, L. Horizontoates A–C: New cholinesterase inhibitors from Cotoneaster horizontalis. Phytochem. Lett. 2014, 10, 204–208. [Google Scholar] [CrossRef]
- Chizzali, C.; Beerhues, L. Phytoalexins of the Pyrinae: Biphenyls and dibenzofurans. Beilstein J. Org. Chem. 2012, 8, 613–620. [Google Scholar] [CrossRef]
- Burden, R.S.; Kemp, M.S.; Wiltshire, C.W. Isolation and structure determination of cotonefuran, an induced antifungal dibenzofuran from Cotoneaster lactea W.W. Sm. J. Chem. Soc. Perkin Trans. 1984, 1, 1445–1448. [Google Scholar] [CrossRef]
- Kokubun, T.; Harborne, J.B.; Eagles, J.; Waterman, P.G. Dibenzofuran phytoalexins from the sapwood of Cotoneaster acutifolius and five related species. Phytochemistry 1995, 38, 57–60. [Google Scholar] [CrossRef]
- Harborne, J.B. The comparative biochemistry of phytoalexin induction in plants. Biochem. Syst. Ecol. 1999, 27, 336–367. [Google Scholar] [CrossRef]
- Vetter, J. Plant cyanogenic glycosides. Toxicon 2000, 38, 11–36. [Google Scholar] [CrossRef]
- Nahrstedt, A. Cyanogenesis in Cotoneaster-arten. Phytochemistry 1973, 12, 1539–1542. [Google Scholar] [CrossRef]
- Sokkar, N.; El-Gindi, O.; Sayed, S.; Mohamed, S.; Ali, Z.; Alfishawy, I. Antioxidant, anticancer and hepatoprotective activities of Cotoneaster horizontalis Decne extract as well as α-tocopherol and amygdalin production from in vitro culture. Acta Physiol. Plant. 2013, 35, 2421–2428. [Google Scholar] [CrossRef]
- Tidwell, R.H.; Beal, J.L.; Patel, D.G.; Tye, A.; Patil, P.N. A study of the cyanogenetic content and toxicity of the fruit of selected species of Cotoneaster. Econ. Bot. 1970, 24, 47–50. [Google Scholar] [CrossRef]
- Bisht, G. Chemical constituents from the fruits of Cotoneaster microphylla Wall ex Lindl. Chem. Asian J. 1995, 7, 455–456. [Google Scholar]
- Khan, S.; Rehman, A.U.; Riaz, N.; Afza, N.; Malik, A. Isolation studies on Cotoneaster racemiflora. J. Chem. Soc. Pakistan 2007, 29, 620–623. [Google Scholar]
- Matthaus, B.; Özcan, M.M. Fatty acid, tocopherol and squalene contents of Rosaceae seed oils. Bot. Stud. 2014, 55, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kanaani, S.; Sani, A.M.; Yaghooti, F. Antibacterial effects and chemical composition of essential oils from Cotoneaster nummularioides pojark and Sonchus arvensis L. leaves extracts on typical food-borne pathogens. Int. J. Biosci. 2015, 6, 357–365. [Google Scholar]
- Fakhri, M.; Davoodi, A.; Parviz, M.; Ghadi, Z.S.; Mousavinasab, S.N.; Farhadi, R.; Azadbakht, M. Characterization and HPLC analysis of Manna from some Cotoneaster species. Int. J. Pharm. Sci. 2017, 8, 5360–5366. [Google Scholar]
- Azadbakht, M.; Pishva, N.; Mohammadi-Samani, S.; Alinejad, F. The effect of purgative manna on the infant jaundice. Iran. J. Pharm. Sci. 2005, 1, 95–100. [Google Scholar]
- Schaefer, H.M.; McGraw, K.; Catoni, C. Birds use fruit colour as honest signal of dietary antioxidant rewards. Funct. Ecol. 2008, 22, 303–310. [Google Scholar]
- Khan, S.; Riaz, N.; Afza, N.; Malik, A.; Rehman, A.U.; Iqbal, L.; Lateef, M. Antioxidant constituents from Cotoneaster racemiflora. J. Asian Nat. Prod. Res. 2009, 11, 44–48. [Google Scholar] [CrossRef]
- Cakilcioglu, U.; Turkoglu, I. An ethnobotanical survey o medicinal plants in Sivrice (Elazığ-Turkey). J. Ethnopharmacol. 2010, 132, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, M.; Mentel, R.; Jha, P.K.; Chaudhary, R.P.; Bhattarai, S.; Gewali, M.B.; Karmacharya, N.; Hipper, M.; Lindequist, U. Antiviral activity of some plants used in nepalese traditional medicine. Evid. Based Complementary Altern. Med. 2009, 6, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, L.; Semotiuk, A.; Zafar, M.; Ahmad, M.; Sultana, S.; Liu, Q.R.; Zada, M.P.; Abidin, S.Z.; Yaseen, G. Ethnopharmacological documentation of medicinal plants used for hypertension among the local communities of DIR Lower, Pakistan. J. Ethnopharmacol. 2015, 175, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, M.; Davoodi, A.; Hamzegardeshi, Z.; Farhadi, R.; Mousavinasab, N.; Keshtkar, A.; Azadbakht, M. Is cotoneaster manna improving the treatment of neonatal jaundice? Bangladesh J. Pharmacol. 2018, 13, 168–178. [Google Scholar] [CrossRef]
- Bukhari, S.A.; Qasim, M.; Masoud, M.S.; Rahman, M.U.; Anwar, H.; Waqas, A.; Mustafa, G. Evaluation of medicinally important constituents of Cotoneaster afghanicus G. Klotz collected from baluchistan region of Pakistan. Indian J. Pharm. Sci. 2019, 81, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jia, J.; Jing, X.; Li, G. Antioxidant activities of extracts from sarcocarp of Cotoneaster multiflorus. J. Chem. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Bernstein, N.; Akram, M.; Daniyal, M.; Koltai, H.; Fridlender, M.; Gorelick, J. Chapter four—Anti-inflammatory potential of medicinal plants: A source for therapeutic secondary metabolites. Adv. Agron. 2018, 150, 131–183. [Google Scholar]
- Zengin, G.; Ferrante, C.; Menghini, L.; Orlando, G.; Brunetti, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Ronci, M.; Aumeeruddy, M.Z.; et al. Protective effects of Cotoneaster integerrimus on in vitro and ex-vivo models of H2O2-induced lactate dehydrogenase activity in HCT116 cell and on lipopolysaccharide-induced inflammation in rat colon. J. Food Biochem. 2019, 43, e12766. [Google Scholar] [CrossRef]
- Yaghooti, F.; Sani, A.M. Antibacterial activity of methanolic extracts from Cotoneaster nummularioides, Cynodon dactylon and Cardaria draba on typical food-borne pathogens. Int. J. Biosci. 2015, 6, 349–356. [Google Scholar]
- Fakhri, M.; Farhadi, R.; Mousavinasab, N.; Hosseinimehr, S.J.; Yousefi, S.S.; Davoodi, A.; Azadbakht, M. Preventive effect of purgative manna on neonatal jaundice: A double blind randomized controlled clinical trial. J. Ethnopharmacol. 2019, 236, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Farhat, A.; Mohammadzadeh, A.; Amiri, M.; Remezani, M. Effect of Cotoneaster tricolor Pajork manna on serum bilirubin levels in neonates. Int. J. Pharmacol. 2006, 2, 455–458. [Google Scholar]
- Rafieian-Kopaei, M.; Khoshdel, A.; Kheiri, S.; Shemian, R. Cotoneaster: A safe and easy way to reduce neonatal jaundice. J. Clin. Diagn. Res. 2016, 10, SC01–SC03. [Google Scholar] [CrossRef] [PubMed]






| Species | Plant Part | Identified Compounds | Identification Method | Phenolic Content | Ref. |
|---|---|---|---|---|---|
| C. serotina | flowers | biochanin A 7-O-glucoside (sissotrin) (22) | isolation; UV, IR | - | [8] |
| C. pannosa | flowers, fruits | biochanin A 7-O-glucoside (sissotrin) (22) | isolation; UV, IR | - | [8] |
| C. oligantha | leafy twigs | isoquercitrin (2), quercetin 3-O-gentiobioside (6) | isolation; UV, IR, acid hydrolysis products | - | [12] |
| leafy twigs | (+)-catechin (23), (-)-epicatechin (24), procyanidin dimer B type | isolation; UV, IR | - | [9] | |
| C. thymaefolia | leaves | quercitrin (4), rutin (5), vitexin (10), vitexin 2″-O-arabinoside (12), vitexin 2″-O-rhamnoside (13); 5,7,2′,5′-tetrahyroxyflavanone (18), 5,7,2′,5′-tetrahyroxyflavanone 7-O-glucoside (19) | isolation; 1H, 13C NMR, FAB-MS, UV, acid hydrolysis products | - | [13] |
| C. simonsii | leafy twigs | 7-methylkaempferol 4′-O-glucoside (9), 5-methylgenistein (20), 5-methylgenistein 4′-O-glucoside (21), (+)-catechin (23) | isolation; 1H, 13C NMR, DEPT, COSY, NOESY, FAB-MS, UV, acid hydrolysis products | - | [14] |
| C. orbicularis | leafy twigs | isoquercitrin (2), hyperoside (3), rutin (5), vitexin (10), vitexin 2″-O-rhamnoside (13), apigenin 6,8-C-dicellobioside (vicenin II 4″,4′′′-O-glucoside) (14), naringenin (17), (+)-catechin (23), (-)-epicatechin (24) | isolation; 1H, 13C NMR, HMBC, FAB-MS, UV, acid hydrolysis products | - | [15] |
| C. horizontalis | leafy twigs | luteolin, quercetin (1), naringenin (17), (+)-catechin (23) | HPLC-PDA | HPLC-PDA: 15.9 mg/g PMFC: 14.0 mg GAE/g ME | [26] |
| leaves | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin 3-O-(2″-O-xylosyl)galactoside (7), quercetin 3-O-glucoside-7-O-rhamnoside, quercetin rhamnoside-hexoside, kaempferol rhamnoside-hexoside, quercetin dirhamnoside, (-)-epicatechin (23), procyanidins B2 (26) and C1 (27), procyanidin tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 73.0 mg GAE/g PM | [19] | |
| fruits | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin 3-O-(2″-O-xylosyl)galactoside (7), quercetin rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 30.5 mg GAE/g PM | [21] | |
| C. acuminatus | roots | apigenin 7-O-glucoside (11), 3′,4′-dihydroxy-6-methoxyflavone 7-O-rhamnoside (15), (-)-epicatechin (24), 7,3′-dihydroxy-4′-methoxyflavanol 5-O-glucoside (25) | isolation; 1H, 13C NMR, FAB-MS, IR, acid hydrolysis products | - | [16] |
| C. melanocarpus | leaves | isoquercitrin (2), hyperoside (3), rutin (5) | HPLC-PDA-MS | - | [5] |
| C. nummularia | leafy twigs | apigenin, luteolin, eriodictyol (16), (+)-catechin (23), (-)-epicatechin (24) | HPLC-PDA | HPLC-PDA: 58.6 mg/g MEFC: 251.8 mg GAE/g ME | [7] |
| C. meyeri | leaves | rutin (5) | HPLC-PDA | HPLC-PDA: 2.2 mg/g PM | [23] |
| C. integerrimus | twigs | quercetin (1), rutin (5), eriodictyol (16), apigenin, hesperidin, (-)-epicatechin (24) | HPLC-PDA | HPLC-PDA: 58.6 mg/g ME; FC: 115.1 mg GAE/g ME | [24] |
| fruits | kaempferol, quercetin (1), rutin (5), (-)-epicatechin (24) | HPLC-PDA | HPLC-PDA: 20.8 mg/g MEFC: 38.5 mg GAE/g ME | [24] | |
| C. integerrimus | fruits | kaempferol, hyperoside (3), rutin (5), (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | HPLC-PDA: 11.4 mg/g PMFC: 49.5 mg GAE/g PM | [22] |
| leaves | quercetin (1), isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), kaempferol hexoside, kaempferol rhamnoside-hexoside, quercetin rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27) | UHPLC-PDA-ESI-MS3 | HPLC-PDA: 48.8 mg/g PMFC: 93.6 mg GAE/g PM HPLC-PDA: 139.0 mg/g ME FC: 241.3 mg GAE/g ME | [19,20,22] | |
| flowers | isoquercitrin (2), hyperoside (3), isorhamnetin hexoside, rutin (5), quercetin rhamnoside-hexoside, (+)-catechin (23), (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | HPLC-PDA: 36.0 mg/g PM FC: 107.0 mg GAE/g PM | [22] | |
| bark | isoquercitrin (2), hyperoside (3), myricetin hexoside, quercetin rhamnoside-hexoside, eriodictyol hexosides (16), (+)-catechin (23), (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | HPLC-PDA: 34.9 mg/g PM FC: 113.6 mg GAE/g PM | [22] | |
| C. bullatus | leaves | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin 3-O-(2″-O-xylosyl)galactoside (7), quercetin rhamnoside-hexoside, quercetin dirhamnoside, (+)-catechin (23), (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer, trimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 154.3 mg GAE/g PM HPLC-PDA: 84.7 mg/g ME FC: 332.9 mg GAE/g ME | [19,20] |
| leaves | hyperoside (3), quercetin 3-O-(2″-O-xylosyl)galactoside (7) | isolation; 1H, 13C NMR, COSY, HMBC, HMQC, ESI-MS, acid hydrolysis products | - | [17] | |
| fruits | isoquercitrin (2), hyperoside (3), quercitrin (4), quercetin 3-O-(2″-O-xylosyl)galactoside (7), quercetin rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 37.3 mg GAE/g PM | [21] | |
| C. zabelii | leaves | quercetin (1), isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin 3-O-glucoside-7-O-rhamnoside, quercetin rhamnoside-hexoside, kaempferol rhamnoside-hexoside, quercetin dirhamnoside, (+)-catechin (23), (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer, trimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 129.4 mg GAE/g PM HPLC-PDA: 69.7 mg/g ME FC: 311.5 mg GAE/g ME | [19,20] |
| leaves | hyperoside (3), quercitrin (4), (-)-epicatechin (24), procyanidin B2 (26) | isolation; 1H, 13C NMR, COSY, HMBC, HMQC, ESI-MS, acid hydrolysis products | - | [17] | |
| fruits | isoquercitrin (2), hyperoside (3), rutin (5), quercetin rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 43.0 mg GAE/g PM | [21] | |
| C. tomentosus | leaves | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin rhamnoside-hexoside, kaempferol rhamnoside-hexoside, quercetin dirhamnoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27) | UHPLC-PDA-ESI-MS3 | FC: 51.7 mg GAE/g PM | [19] |
| C. melanocarpus | leaves | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), kaempferol rhamnoside-hexoside, quercetin rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27) | UHPLC-PDA-ESI-MS3 | FC: 54.8 mg GAE/g PM | [19] |
| C. lucidus | leaves | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin rhamnoside-hexoside, kaempferol rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins trimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 106.8 mg GAE/g PM | [19] |
| C. lucidus | fruits | isoquercitrin (2), hyperoside (3), rutin (5), quercetin rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidin dimer B-type | UHPLC-PDA-ESI-MS3 | FC: 28.7 mg GAE/g PM | [21] |
| C. divaricatus | leaves | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin rhamnoside-hexoside, kaempferol rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer, trimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 119.7 mg GAE/g PM | [19] |
| fruits | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidin dimer B-type | UHPLC-PDA-ESI-MS3 | FC: 29.7 mg GAE/g PM | [21] | |
| C. nanshan | leaves | isoquercitrin (2), hyperoside (3), rutin (5), quercetin 3-O-(2″-O-xylosyl)galactoside (7), quercetin rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidin tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 84.3 mg GAE/g PM | [19] |
| fruits | isoquercitrin (2), hyperoside (3), rutin (5), (-)-epicatechin (24), procyanidin B2 (26), procyanidin dimer B-type | UHPLC-PDA-ESI-MS3 | FC: 26.0 mg GAE/g PM | [21] | |
| C. hjelmqvistii | leaves | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin 3-O-(2″-O-xylosyl)galactoside (7), quercetin rhamnoside-hexoside, kaempferol rhamnoside-hexoside, (-)-epicatechin (24),procyanidins B2 (26) and C1 (27), procyanidins dimer, trimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 124.9 mg GAE/g PM | [19] |
| fruits | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin 3-O-(2″-O-xylosyl)galactoside (7), quercetin rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 43.5 mg GAE/g PM | [21] | |
| C. dielsianus | leaves | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin 3-O-(2″-O-xylosyl)galactoside (7), quercetin rhamnoside-hexoside, kaempferol rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidin tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 69.9 mg GAE/g PM | [19] |
| fruits | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin 3-O-(2″-O-xylosyl)galactoside (7), quercetin rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 31.0 mg GAE/g PM | [21] | |
| C. splendens | leaves | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin 3-O-(2″-O-xylosyl)galactoside (7), quercetin rhamnoside-hexoside, kaempferol rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer, trimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 99.2 mg GAE/g PM | [19] |
| fruits | isoquercitrin (2), hyperoside (3), quercitrin (4), rutin (5), quercetin 3-O-(2″-O-xylosyl)galactoside (7), quercetin rhamnoside-hexoside, (-)-epicatechin (24), procyanidins B2 (26) and C1 (27), procyanidins dimer and tetramer B-type | UHPLC-PDA-ESI-MS3 | FC: 38.5 mg GAE/g PM | [21] | |
| C. pannosus | fruits | hyperoside (3), rutin (5), naringenin (17) | HPLC-PDA | HPLC-PDA: 4.0 mg/g PM | [25] |
| C. mongolica | leaves | quercetin (1), hyperoside (3), astragalin (8), biochanin A 7-O-glucoside (sissotrin) (22) | isolation; 1H, 13C NMR, acid hydrolysis products | - | [18] |
| Species | Plant Part/Extract or Fraction | Antioxidant Assay | Antioxidant Effect | Ref. |
|---|---|---|---|---|
| C. horizontalis | leafy twigs/ethanolic extract | DPPH | EC50 = 19.3 µg/mL | [35] |
| C. melanocarpus | young and old twigs, leaves/ aqueous, methanolic, ethyl acetate, dichloromethane and hexane extracts | DPPH | aqueous extracts: EC50 = 53.5–86.7 µg/mL methanolic extracts: EC50 = 30.9–106.4 µg/mL ethyl acetate extracts: EC50 = 74.9–>200 µg/mL dichloromethane extracts: EC50 = 134.6–>200 µg/mL hexane extracts: EC50 = no activity | [5] |
| leaves/methanol-water (7:3, v/v) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 32.75 µg/mL PM; O2•−: EC50 = 31.59 µg/mL PM H2O2: EC50 = 56.80 µg/mL PM; FRAP = 0.95 mmol Fe2+/g PM | [19] | |
| C. nummularia | leafy twigs/aqueous, methanolic, ethyl acetate extracts | DPPH, ABTS, O2•−, FRAP, CUPRAC, phosphomolibdenum assay, β-carotene/linoleic acid assay, metal chelating assay | DPPH: EC50 = 0.097–0.252 mg/mL; ABTS: EC50 = 0.020–0.043 mg/mL; O2•−: EC50 = 1.066–1.603 mg/mL; FRAP = 0.143–0.654 mg/mL; CUPRAC: EC50 = 0.022–0.263 mg/mL phosphomolibdenum assay: EC50 = 56.1–177.3 mg AE/g β-carotene/linoleic acid assay: 85.5–93.0% (2 mg/mL; the extract concn.) metal chelating assay: EC50 = 0.3–18.7 mg EDTAE/g | [7] |
| leaves/ethanolic extract | DMPD, metal chelating assay | DMPD: 20.9% (2 mg/mL; the extract concn.) metal chelating assay: 26.2% (2 mg/mL; the extract concn.) | [23] | |
| C. meyeri | leaves/ethanolic extract | DMPD, metal chelating assay | DMPD: 8.2% (2 mg/mL; the extract concn.) metal chelating assay: 5.9% (2 mg/mL; the extract concn.) | [23] |
| C. morulus | leaves/ethanolic extract | DMPD, metal chelating assay | DMPD: 11.2% (2 mg/mL; the extract concn.) metal chelating assay: 21.5% (2 mg/mL; the extract concn.) | [23] |
| C. integerrimus | twigs/aqueous, methanolic extracts | DPPH, FRAP, CUPRAC, phosphomolibdenum assay, metal chelating assay | DPPH: EC50 = 1.06–1.09 mg/mL; FRAP = 257.4–265.4µg/mL; CUPRAC: EC50 = 336.5–349.0 µg/mL phosphomolibdenum assay: EC50 = 0.36–0.53 mg/mL metal chelating assay: EC50 = 1.47–6.24 mg/mL | [24] |
| fruits/aqueous, methanolic extracts | DPPH, FRAP, CUPRAC, phosphomolibdenum assay, metal chelating assay | DPPH: EC50 = 1.85–2.44 mg/mL; FRAP = 376.4–498.8µg/mL; CUPRAC: EC50 = 596.2–657.4 µg/mL phosphomolibdenum assay: EC50 = 1.24–1.38 mg/mL metal chelating assay: EC50 = 2.14–6.14 mg/mL | [24] | |
| leaves/methanol-water (7:3) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 24.58 µg/mL PM; O2•−: EC50 = 29.49 µg/mL PM; H2O2: EC50 = 55.42 µg/mL PM; FRAP= 2.04 mmol Fe2+/g PM | [19] | |
| C. integerrimus | leaves/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 23.80 µg/mL PM; FRAP: 2.77 mmol Fe2+/g PM; TBARS: IC50 = 23.19 µg/mL PM | [22] |
| leaves/methanol-water (7:3) extract and diethyl ether, ethyl acetate, n-butanol and water fractions prepared by fractionated extraction | DPPH, FRAP | DPPH: EC50 = 5.19–27.88 µg/mL; FRAP: 3.82–15.84 mmol Fe2+/g | [20] | |
| flowers/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 20.65µg/mL PM; FRAP: 2.82 mmol Fe2+/g PM; TBARS: IC50 = 28.89 µg/mL PM | [22] | |
| bark/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 20.76 µg/mL PM; FRAP: 2.75 mmol Fe2+/g PM; TBARS: IC50 = 26.01 µg/mL PM | [22] | |
| fruits/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 54.17 µg/mL PM; FRAP: 1.10 mmol Fe2+/g PM; TBARS: IC50 = 47.32 µg/mL PM | [22] | |
| C. tomentosus | leaves/methanol-water (7:3) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 34.50 µg/mL PM; O2•−: EC50 = 74.79 µg/mL PM; H2O2: EC50 = 91.30 µg/mL PM; FRAP= 0.90 mmol Fe2+/g PM | [19] |
| C. lucidus | leaves/methanol-water (7:3) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 25.35 µg/mL PM; O2•−: EC50 = 27.70 µg/mL PM; H2O2: EC50 = 38.46 µg/mL PM; FRAP= 2.18 mmol Fe2+/g PM | [19] |
| fruits/methanol-water (7: 3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 123.41µg/mL PM; FRAP: 0.70 mmol Fe2+/g PM; TBARS: IC50 = 108.70 µg/mL PM | [21] | |
| C. divaricatus | leaves/methanol-water (7:3) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 18.45 µg/mL PM; O2•−: EC50 = 37.57 µg/mL PM; H2O2: EC50 = 36.06 µg/mL PM; FRAP= 2.39 mmol Fe2+/g PM | [19] |
| fruits/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 91.47 µg/mL PM; FRAP: 0.76 mmol Fe2+/g PM; TBARS: IC50 = 83.16 µg/mL PM | [21] | |
| C. horizontalis | leaves/methanol-water (7:3) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 23.02 µg/mL PM; O2•−: EC50 = 49.48 µg/mL PM; H2O2: EC50 = 71.14 µg/mL PM; FRAP= 2.08 mmol Fe2+/g PM | [19] |
| fruits/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 93.32 µg/mL PM; FRAP: 0.85 mmol Fe2+/g PM; TBARS: IC50 = 84.89 µg/mL PM | [21] | |
| C. nanshan | leaves/methanol-water (7:3) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 24.68 µg/mL PM; O2•−: EC50 = 55.74 µg/mL PM; H2O2: EC50 = 54.17 µg/mL PM; FRAP= 1.70 mmol Fe2+/g PM | [19] |
| fruits/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 178.35 µg/mL PM; FRAP: 0.61 mmol Fe2+/g PM; TBARS: IC50 = 165.76 µg/mL PM | [21] | |
| C. hjelmqvistii | leaves/methanol-water (7:3) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 21.04 µg/mL PM; O2•−: EC50 = 28.10 µg/mL PM; H2O2: EC50 = 35.57 µg/mL PM; FRAP= 2.59 mmol Fe2+/g PM | [19] |
| fruits/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 64.51 µg/mL PM; FRAP: 1.05 mmol Fe2+/g PM; TBARS: IC50 = 62.96 µg/mL PM | [21] | |
| C. dielsianus | leaves/methanol-water (7:3) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 29.49 µg/mL PM; O2•−: EC50 = 54.50µg/mL PM; H2O2: EC50 = 62.97 µg/mL PM; FRAP= 1.71 mmol Fe2+/g PM | [19] |
| fruits/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 117.10 µg/mL PM; FRAP: 0.67 mmol Fe2+/g PM; TBARS: IC50 = 103.72 µg/mL PM | [21] | |
| C. splendens | leaves/methanol-water (7:3) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 22.56 µg/mL PM; O2•−: EC50 = 44.07 µg/mL PM; H2O2: EC50 = 43.24 µg/mL PM; FRAP= 2.44 mmol Fe2+/g PM | [19] |
| fruits/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 67.15 µg/mL PM; FRAP: 0.98 mmol Fe2+/g PM; TBARS: IC50 = 66.21 µg/mL PM | [21] | |
| C. bullatus | leaves/methanol-water (7:3) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 20.91 µg/mL PM; O2•−: EC50 = 37.80 µg/mL PM; H2O2: EC50 = 28.95 µg/mL PM; FRAP= 3.76 mmol Fe2+/g PM | [19] |
| leaves/methanol-water (7:3) extract and diethyl ether, ethyl acetate, n-butanol and water fractions prepared by fractionated extraction | DPPH, FRAP | DPPH: EC50 = 3.19–22.41 µg/mL; FRAP: 3.99–16.99 mmol Fe2+/g | [20] | |
| fruits/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 66.31 µg/mL PM; FRAP: 0.97 mmol Fe2+/g PM; TBARS: IC50 = 64.99 µg/mL PM | [21] | |
| C. zabelii | leaves/methanol-water (7:3) extract | DPPH, O2•−, H2O2, FRAP | DPPH: EC50 = 21.52 µg/mL PM; O2•−: EC50 = 52.76 µg/mL PM; H2O2: EC50 = 43.28 µg/mL PM; FRAP= 2.98 mmol Fe2+/g PM | [19] |
| leaves/methanol-water (7:3) extract and diethyl ether, ethyl acetate, n-butanol and water fractions prepared by fractionated extraction | DPPH, FRAP | DPPH: EC50 = 3.95–18.73 µg/mL; FRAP: 3.89–16.74 mmol Fe2+/g | [20] | |
| fruits/methanol-water (7:3) extract | DPPH, FRAP, TBARS | DPPH: EC50 = 62.93 µg/mL PM; FRAP: 1.09 mmol Fe2+/g PM; TBARS: IC50 = 62.54µg/mL PM | [21] | |
| C. mongolica | leaves/methanolic extract and chloroform, n-butanol and water fractions prepared by fractionated extraction | DPPH | EC50 = 55.7–>200 µg/mL | [18] |
| fruits/ methanolic extract and n-butanol fraction prepared by fractionated extraction | DPPH | EC50 = 108.5–>200 µg/mL | [18] | |
| C. pannosus | fruits/ethanol:water (7:3), ethanol hexane (55:45) extracts | DPPH | EC50 = 47.3–54.9 µg/mL | [25] |
| C. afghanicus | leafy twigs/hexane, ethanolic extracts | DPPH | EC50 = 57.4–64.7 µg/mL | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicel, A. An Overview of the Genus Cotoneaster (Rosaceae): Phytochemistry, Biological Activity, and Toxicology. Antioxidants 2020, 9, 1002. https://doi.org/10.3390/antiox9101002
Kicel A. An Overview of the Genus Cotoneaster (Rosaceae): Phytochemistry, Biological Activity, and Toxicology. Antioxidants. 2020; 9(10):1002. https://doi.org/10.3390/antiox9101002
Chicago/Turabian StyleKicel, Agnieszka. 2020. "An Overview of the Genus Cotoneaster (Rosaceae): Phytochemistry, Biological Activity, and Toxicology" Antioxidants 9, no. 10: 1002. https://doi.org/10.3390/antiox9101002