Radioprotective Role of Peroxiredoxin 6
Abstract
:1. Introduction
2. Regulation of PRDX6 Expression
3. Role of Endogenous Prdxs in Radioresistance of Mammalian Cells
4. Application of Exogenous Prdx6 as a Radioprotector
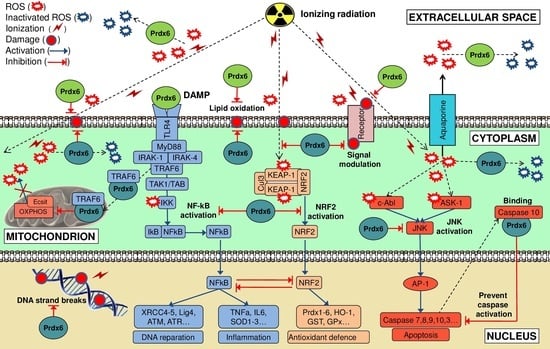
5. Molecular Mechanisms of Radioprotective Effect of Endogenous and Exogenous Prdx6
5.1. Endogenous Prdx6
5.2. Exogenous Prdx6
6. Practical Aspects of Prdx6 Radioprotective Action
6.1. Prdx6 Suppression in Treatment of Radioresistant Cancer
6.2. Prdx6 Application as a Radioprotective Agent
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sankaranarayanan, K. Estimation of the hereditary risks of exposure to ionizing radiation: History, current status, and emerging perspectives. Heal. Phys. 2001, 80, 363–369. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, 14S–22S. [Google Scholar] [CrossRef]
- Ward, J.F. DNA Damage Produced by Ionizing Radiation in Mammalian Cells: Identities, Mechanisms of Formation, and Reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988. [Google Scholar] [CrossRef]
- Konings, A.W.T.; Drijver, E.B. Radiation effects on membranes. I. Vitamin E deficiency and lipid peroxidation. Peroxidation. Radiat. Res. 1979, 80, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Bruskov, V.I.; Karp, O.E.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Gudkov, S.V. Prolongation of oxidative stress by long-lived reactive protein species induced by X-ray radiation and their genotoxic action. Free Radic. Res. 2012, 46, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, A.V.; Gudkov, S.V.; Usacheva, A.M.; Bruskov, V.I. Exogenous 8-Oxo-7,8-dihydro-2′-deoxyguanosine: Biomedical Properties, Mechanisms of Action, and Therapeutic Potential. Biochemistry 2017, 82, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Lankin, V.Z.; Tikhaze, A.K.; Kapel’ko, V.I.; Shepel’kova, G.S.; Shumaev, K.B.; Panasenko, O.M.; Konovalova, G.G.; Belenkov, Y.N. Mechanisms of oxidative modification of low density lipoproteins under conditions of oxidative and carbonyl stress. Biochemistry 2007, 72, 1081–1090. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Tikhaze, A.K. Role of Oxidative Stress in the Genesis of Atherosclerosis and Diabetes Mellitus: A Personal Look Back on 50 Years of Research. Curr. Aging Sci. 2017. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Riley, P.A. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.-L. Oxidatively generated base damage to cellular DNA. Free Radic. Biol. Med. 2010, 49, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Shilyagina, N.Y.; Vodeneev, V.A.; Zvyagin, A.V. Targeted Radionuclide Therapy of Human Tumors. Int. J. Mol. Sci. 2016, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.F.; Landauer, M.R. Radioprotection by antioxidants. Ann. N. Y. Acad. Sci. 2000, 899, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Kostevich, V.A.; Varfolomeeva, E.Y.; Grigorieva, D.V.; Gorudko, I.V.; Kozlov, S.O.; Kudryavtsev, I.V.; Mikhalchik, E.V.; Filatov, M.V.; Cherenkevich, S.N.; et al. Capacity of ceruloplasmin to scavenge products of the respiratory burst of neutrophils is not altered by the products of reactions catalyzed by myeloperoxidase. Biochem. Cell Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Ravin, V.K.; Novoselov, V.I. Peroxiredoxins as multifunctional enzymes. Mol. Biol. (Mosk) 2014, 48, 600–628. [Google Scholar] [CrossRef] [PubMed]
- Dubbs, J.M.; Mongkolsuk, S. Peroxiredoxins in bacterial antioxidant defense. Subcell. Biochem. 2007, 44, 143–193. [Google Scholar] [CrossRef]
- Park, S.G.; Cha, M.K.; Jeong, W.; Kim, I.H. Distinct physiological functions of thiol peroxidase isoenzymes in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 5723–5732. [Google Scholar] [CrossRef]
- Rhee, S.G. Overview on Peroxiredoxin. Mol. Cells 2016, 39, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Dietz, K.-J. Peroxiredoxins in plants and cyanobacteria. Antioxid. Redox Signal. 2011, 15, 1129–1159. [Google Scholar] [CrossRef]
- Knoops, B.; Loumaye, E.; Van der Eecken, V. Evolution of the peroxiredoxins. Peroxiredoxin Syst. 2007. [Google Scholar] [CrossRef]
- Rhee, S.G.; Kil, I.S. Multiple Functions and Regulation of Mammalian Peroxiredoxins. Annu. Rev. Biochem. 2016, 85, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.A.; Krause, D.S.; Carman, C.V.; Das, S.; Dubey, D.P.; Abraham, J.L.; Bronson, R.T.; Fujiwara, Y.; Orkin, S.H.; Van Etten, R.A. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 2003, 424, 561–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londoño-Vallejo, J.A. Telomere instability and cancer. Biochimie 2008, 90, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Maciejowski, J.; de Lange, T. Telomeres in cancer: Tumour suppression and genome instability. Nat. Rev. Mol. Cell Biol. 2017, 18, 175–186. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, S.U.; Yu, S.L.; Kim, S.H.; Park, D.S.; Moon, H.B.; Dho, S.H.; Kwon, K.S.; Kwon, H.J.; Han, Y.H.; et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 2003, 101, 5033–5038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wonsey, D.R.; Zeller, K.I.; Dang, C.V. The c-Myc target gene PRDX3 is required for mitochondrial homeostasis and neoplastic transformation. Proc. Natl. Acad. Sci. USA 2002, 99, 6649–6654. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-G.; Wang, L.; Kaifu, T.; Li, J.; Li, X.; Li, L. Featured Article: Accelerated decline of physical strength in peroxiredoxin-3 knockout mice. Exp. Biol. Med. 2016, 241, 1395–1400. [Google Scholar] [CrossRef] [Green Version]
- Iuchi, Y.; Okada, F.; Tsunoda, S.; Kibe, N.; Shirasawa, N.; Ikawa, M.; Okabe, M.; Ikeda, Y.; Fujii, J. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem. J. 2009, 419, 149–158. [Google Scholar] [CrossRef]
- Wang, X.; Phelan, S.A.; Forsman-Semb, K.; Taylor, E.F.; Petros, C.; Brown, A.; Lerner, C.P.; Paigen, B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J. Biol. Chem. 2003, 278, 25179–25190. [Google Scholar] [CrossRef]
- Perkins, A.; Poole, L.B.; Karplus, P.A. Tuning of peroxiredoxin catalysis for various physiological roles. Biochemistry 2014, 53, 7693–7705. [Google Scholar] [CrossRef] [PubMed]
- Peshenko, I.V.; Singh, A.K.; Shichi, H. Bovine eye 1-Cys peroxiredoxin: Expression in E. coli and antioxidant properties. J. Ocul. Pharmacol. Ther. 2001, 17, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Vasquez-Medina, J.P.; Dodia, C.; Sorokina, E.M.; Tao, J.-Q.; Feinstein, S.I. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2018, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Redox signaling across cell membranes. Antioxid. Redox Signal. 2009, 11, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid. Redox Signal. 2011, 15, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jo, H.-Y.; Kim, M.H.; Cha, Y.-Y.; Choi, S.W.; Shim, J.-H.; Kim, T.J.; Lee, K.-Y. H2O2-dependent hyperoxidation of peroxiredoxin 6 (Prdx6) plays a role in cellular toxicity via up-regulation of iPLA2 activity. J. Biol. Chem. 2008, 283, 33563–33568. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. The phospholipase A2 activity of peroxiredoxin 6. J. Lipid Res. 2018, 59, 1132–1147. [Google Scholar] [CrossRef]
- Fisher, A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017, 617, 68–83. [Google Scholar] [CrossRef]
- Bast, A.; Erttmann, S.F.; Walther, R.; Steinmetz, I. Influence of iNOS and COX on peroxiredoxin gene expression in primary macrophages. Free Radic. Biol. Med. 2010, 49, 1881–1891. [Google Scholar] [CrossRef]
- Kim, H.S.; Kang, S.W.; Rhee, S.G.; Clerch, L.B. Rat lung peroxiredoxins I and II are differentially regulated during development and by hyperoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L1212–L1217. [Google Scholar] [CrossRef]
- Kim, H.-S.; Manevich, Y.; Feinstein, S.I.; Pak, J.H.; Ho, Y.S.; Fisher, A.B. Induction of 1-cys peroxiredoxin expression by oxidative stress in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L363–L369. [Google Scholar] [CrossRef]
- Das, K.C.; Pahl, P.M.; Guo, X.L.; White, C.W. Induction of peroxiredoxin gene expression by oxygen in lungs of newborn primates. Am. J. Respir. Cell. Mol. Biol. 2001, 25, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kimura, S.; Seto, K.; Warabi, E.; Kawachi, Y.; Shoda, J.; Tabuchi, K.; Yamagata, K.; Hasegawa, S.; Bukawa, H.; et al. Peroxiredoxin I plays a protective role against UVA irradiation through reduction of oxidative stress. J. Dermatol. Sci. 2014, 74, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, J.S.; Kim, Y.J.; Soo Lee, Y.; Sook Hwang, T.; Kim, D.J.; Park, E.M.; Park, Y.M. Differential expression of Prx I and II in mouse testis and their up-regulation by radiation. Biochem. Biophys. Res. Commun. 2002, 296, 337–342. [Google Scholar] [CrossRef]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory Mechanisms Controlling Gene Expression Mediated By the Antioxidant Response Element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16209. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, K.; Fujikawa, N.; Komatsu, M.; Ishii, T.; Unno, M.; Akaike, T.; Motohashi, H.; Yamamoto, M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 13561–13566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, A.; Wijayanti, N.; Neuschäfer-Rube, A.P.; Katz, N.; Kietzmann, T.; Immenschuh, S. Phorbol ester-dependent activation of peroxiredoxin I gene expression via a protein kinase C, Ras, p38 mitogen-activated protein kinase signaling pathway. J. Biol. Chem. 2003, 278, 45419–45434. [Google Scholar] [CrossRef]
- Egler, R.A.; Fernandes, E.; Rothermund, K.; Sereika, S.; de Souza-Pinto, N.; Jaruga, P.; Dizdaroglu, M.; Prochownik, E. V Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene 2005, 24, 8038–8050. [Google Scholar] [CrossRef]
- Quan, C.; Cha, E.-J.; Lee, H.-L.; Han, K.H.; Lee, K.M.; Kim, W.-J. Enhanced expression of peroxiredoxin I and VI correlates with development, recurrence and progression of human bladder cancer. J. Urol. 2006, 175, 1512–1516. [Google Scholar] [CrossRef]
- Riddell, J.R.; Maier, P.; Sass, S.N.; Moser, M.T.; Foster, B.A.; Gollnick, S.O. Peroxiredoxin 1 stimulates endothelial cell expression of VEGF via TLR4 dependent activation of HIF-1α. PLoS ONE 2012, 7, e50394. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Chen, I.; Chen, Y.; Alkam, D.; Wang, Y.; Semenza, G.L. PRDX2 and PRDX4 are negative regulators of hypoxia-inducible factors under conditions of prolonged hypoxia. Oncotarget 2016, 7, 6379–6397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, H.; Gao, Y.-H.; Han, D.-Y.; Li, Q.-Y.; Feng, L.-J.; Zhang, W.; Ji, G.; Xiao, J.-C.; Zhang, H.-Z.; Wei, Q. Hypoxia inducible factor-1α suppresses Peroxiredoxin 3 expression to promote proliferation of CCRCC cells. FEBS Lett. 2014, 588, 3390–3394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, F.; Goossens, E.; Atallah, N.M.; Grimard, M.; Kelley, M.R.; Fishel, M.L. APE1/Ref-1 knockdown in pancreatic ductal adenocarcinoma—Characterizing gene expression changes and identifying novel pathways using single-cell RNA sequencing. Mol. Oncol. 2017, 11, 1711–1732. [Google Scholar] [CrossRef]
- Gallagher, B.M.; Phelan, S.A. Investigating transcriptional regulation of Prdx6 in mouse liver cells. Free Radic. Biol. Med. 2007, 42, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.H.; Son, W.C.; Seo, S.B.; Hong, S.J.; Sohn, W.M.; Na, B.K.; Kim, T.S. Peroxiredoxin 6 expression is inversely correlated with nuclear factor-κB activation during Clonorchis sinensis infestation. Free Radic. Biol. Med. 2016, 99, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Cong, N.; Huang, W.; Yuan, J.-P.; Li, G.-Z.; Zhai, G.-S.; Li, B.-S. Peroxiredoxin1 promotes cell proliferation, migration and invasion of colorectal cancer via p38MAPK signaling. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1922–1928. [Google Scholar]
- Sun, H.; Feng, L.; Wang, A.; Wang, J.; Liu, L.; Jin, M.; Shen, G.; Jin, C.; Lee, D.; Kwon, T.; et al. Peroxiredoxin I deficiency increases LPS-induced lethal shock in mice. Mol. Med. Rep. 2018, 18, 2427–2432. [Google Scholar] [CrossRef]
- Immenschuh, S.; Stritzke, J.; Iwahara, S.; Ramadori, G. Up-regulation of heme-binding protein 23 (HBP23) gene expression by lipopolysaccharide is mediated via a nitric oxide-dependent signaling pathway in rat Kupffer cells. Hepatology 1999, 30, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.-Y.; Shi, J.-X.; Shi, S.-L.; Liu, F.; Rui, G.; Li, X.; Gao, L.-B.; Deng, X.-L.; Li, Q.-F. Nucleophosmin Regulates Intracellular Oxidative Stress Homeostasis via Antioxidant PRDX6. J. Cell. Biochem. 2017, 118, 4697–4707. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.J.; Mills, J.N.; Bandurraga, S.G.; Nogueira, L.M.; Mason, N.J.; Camp, E.R.; Larue, A.C.; Turner, D.P.; Findlay, V.J. MicroRNA-510 promotes cell and tumor growth by targeting peroxiredoxin1 in breast cancer. Breast Cancer Res. 2013, 15, R70. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.L.; Nadler, M.; Skoko, J.J.; Bertomeu, T.; Pelosi, A.; Shafaei, P.M.; Levine, K.; Schempf, A.; Pennarun, B.; Yang, B.; et al. A Peroxidase Peroxiredoxin 1-Specific Redox Regulation of the Novel FOXO3 microRNA Target let-7. Antioxid. Redox Signal. 2018, 28, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Joris, V.; Gomez, E.L.; Menchi, L.; Lobysheva, I.; Di Mauro, V.; Esfahani, H.; Condorelli, G.; Balligand, J.-L.; Catalucci, D.; Dessy, C. MicroRNA-199a-3p and MicroRNA-199a-5p Take Part to a Redundant Network of Regulation of the NOS (NO Synthase)/NO Pathway in the Endothelium. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, N.; Wei, H.; Li, C.; Wu, J.; Yang, G. miR-24-3p Regulates Progression of Gastric Mucosal Lesions and Suppresses Proliferation and Invasiveness of N87 Via Peroxiredoxin 6. Dig. Dis. Sci. 2016, 61, 3486–3497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Wang, W.; Gu, Q.; Xue, J.; Cao, H.; Tang, Y.; Xu, X.; Cao, J.; Zhou, J.; Wu, J.; et al. Protein and miRNA profiling of radiation-induced skin injury in rats: The protective role of peroxiredoxin-6 against ionizing radiation. Free Radic. Biol. Med. 2014, 69, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Su, Y.; Ai, G.; Wang, Y.; Wang, T.; Wang, F.; Ionizing, P.I. Involvement of peroxiredoxin I in protecting cells from radiation-induced death. J. Radiat. Res. 2005, 46, 305–312. [Google Scholar] [CrossRef]
- Chen, W.-C.; McBride, W.H.; Iwamoto, K.S.; Barber, C.L.; Wang, C.-C.; Oh, Y.-T.; Liao, Y.-P.; Hong, J.-H.; de Vellis, J.; Shau, H. Induction of radioprotective peroxiredoxin-I by ionizing irradiation. J. Neurosci. Res. 2002, 70, 794–798. [Google Scholar] [CrossRef] [Green Version]
- Miura, Y.; Kano, M.; Yamada, M.; Nishine, T.; Urano, S.; Suzuki, S.; Endo, T.; Toda, T. Proteomic study on X-irradiation-responsive proteins and ageing: Search for responsible proteins for radiation adaptive response. J. Biochem. 2007, 142, 145–155. [Google Scholar] [CrossRef]
- An, J.H.; KIM, J.; Seong, J. Redox signaling by ionizing radiation in mouse liver. Ann. N. Y. Acad. Sci. 2004, 1030, 86–94. [Google Scholar] [CrossRef]
- An, J.H.; Seong, J.S. Proteomics analysis of apoptosis-regulating proteins in tissues with different radiosensitivity. J. Radiat. Res. 2006, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Cerda, M.B.; Lloyd, R.; Batalla, M.; Giannoni, F.; Casal, M.; Policastro, L. Silencing peroxiredoxin-2 sensitizes human colorectal cancer cells to ionizing radiation and oxaliplatin. Cancer Lett. 2017, 388, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Diaz, A.J.G.; Yen, Y. The role of peroxiredoxin II in chemoresistance of breast cancer cells. Breast Cancer Targets Ther. 2014, 6, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.J.G.; Tamae, D.; Yen, Y.; Li, J.; Wang, T. Enhanced radiation response in radioresistant MCF-7 cells by targeting peroxiredoxin II. Breast Cancer Targets Ther. 2013, 5, 87–101. [Google Scholar] [CrossRef]
- Kim, T.H.; Song, J.; Kim, S.H.; Parikh, A.K.; Mo, X.; Palanichamy, K.; Kaur, B.; Yu, J.; Yoon, S.O.; Nakano, I.; et al. Piperlongumine treatment inactivates peroxiredoxin 4, exacerbates endoplasmic reticulum stress, and preferentially kills high-grade glioma cells. Neuro Oncol. 2014, 16, 1354–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Song, J.; Alcantara Llaguno, S.R.; Murnan, E.; Liyanarachchi, S.; Palanichamy, K.; Yi, J.-Y.; Viapiano, M.S.; Nakano, I.; Yoon, S.O.; et al. Suppression of peroxiredoxin 4 in glioblastoma cells increases apoptosis and reduces tumor growth. PLoS ONE 2012, 7, e42818. [Google Scholar] [CrossRef]
- Park, C.-K.; Kim, J.H.; Moon, M.J.; Jung, J.H.; Lim, S.-Y.; Park, S.-H.; Kim, J.-H.; Kim, D.G.; Jung, H.-W.; Cho, B.-K.; et al. Investigation of molecular factors associated with malignant transformation of oligodendroglioma by proteomic study of a single case of rapid tumor progression. J. Cancer Res. Clin. Oncol. 2008, 134, 255–262. [Google Scholar] [CrossRef]
- Lehtonen, S.T.; Svensk, A.-M.; Soini, Y.; Pääkkö, P.; Hirvikoski, P.; Kang, S.W.; Säily, M.; Kinnula, V.L. Peroxiredoxins, a novel protein family in lung cancer. Int. J. Cancer 2004, 111, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.-M.; Park, K.-R.; Lee, H.P.; Lee, D.H.; Jo, M.; Shin, D.H.; Yoon, D.-Y.; Han, S.B.; Hong, J.T. PRDX6 promotes lung tumor progression via its GPx and iPLA2 activities. Free Radic. Biol. Med. 2014, 69, 367–376. [Google Scholar] [CrossRef]
- Yun, H.-M.; Park, K.-R.; Park, M.H.; Kim, D.H.; Jo, M.R.; Kim, J.Y.; Kim, E.-C.; Yoon, D.Y.; Han, S.B.; Hong, J.T. PRDX6 promotes tumor development via the JAK2/STAT3 pathway in a urethane-induced lung tumor model. Free Radic. Biol. Med. 2015, 80, 136–144. [Google Scholar] [CrossRef]
- Chang, X.-Z.; Li, D.-Q.; Hou, Y.-F.; Wu, J.; Lu, J.-S.; Di, G.-H.; Jin, W.; Ou, Z.-L.; Shen, Z.-Z.; Shao, Z.-M. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Karihtala, P.; Mäntyniemi, A.; Kang, S.W.; Kinnula, V.L.; Soini, Y. Peroxiredoxins in breast carcinoma. Clin. Cancer Res. 2003, 9, 3418–3424. [Google Scholar] [PubMed]
- Fujita, Y.; Nakanishi, T.; Hiramatsu, M.; Mabuchi, H.; Miyamoto, Y.; Miyamoto, A.; Shimizu, A.; Tanigawa, N. Proteomics-based approach identifying autoantibody against peroxiredoxin VI as a novel serum marker in esophageal squamous cell carcinoma. Clin. Cancer Res. 2006, 12, 6415–6420. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, I.; Nagata, M.; Takiguchi, N.; Nabeya, Y.; Ikeda, A.; Yokoi, S.; Kuwajima, A.; Tagawa, M.; Matsushita, K.; Satoshi, Y.; et al. Panel of autoantibodies against multiple tumor-associated antigens for detecting gastric cancer. Cancer Sci. 2017, 108, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Chang, J.-W.; Jung, Y.-K. Peroxiredoxin 6 interferes with TRAIL-induced death-inducing signaling complex formation by binding to death effector domain caspase. Cell. Death Differ. 2011, 18, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.; Pearl, A.; Suchy, S.; Tartaglio, J.; Visco, K.; Phelan, S.A. Overexpression of Prdx6 and resistance to peroxide-induced death in Hepa1-6 cells: Prdx suppression increases apoptosis. Redox Rep. 2009, 14, 275–284. [Google Scholar] [CrossRef]
- Pak, J.H.; Choi, W.H.; Lee, H.M.; Joo, W.-D.; Kim, J.-H.; Kim, Y.-T.; Kim, Y.-M.; Nam, J.-H. Peroxiredoxin 6 overexpression attenuates cisplatin-induced apoptosis in human ovarian cancer cells. Cancer Investig. 2011, 29, 21–28. [Google Scholar] [CrossRef]
- Raatikainen, S.; Aaaltomaa, S.; Kärjä, V.; Soini, Y.; Karja, V.; Soini, Y. Increased Peroxiredoxin 6 Expression Predicts Biochemical Recurrence in Prostate Cancer Patients After Radical Prostatectomy. Anticancer Res. 2015, 35, 6465–6470. [Google Scholar]
- Schmitt, A.; Schmitz, W.; Hufnagel, A.; Schartl, M.; Meierjohann, S. Peroxiredoxin 6 triggers melanoma cell growth by increasing arachidonic acid-dependent lipid signalling. Biochem. J. 2015, 471, 267–279. [Google Scholar] [CrossRef]
- Chen, M.-F.; Keng, P.C.; Shau, H.; Wu, C.-T.; Hu, Y.-C.; Liao, S.-K.; Chen, W.-C. Inhibition of lung tumor growth and augmentation of radiosensitivity by decreasing peroxiredoxin I expression. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 581–591. [Google Scholar] [CrossRef]
- Li, G.; Xie, B.; Li, X.; Chen, Y.; Xu, Y.; Xu-Welliver, M.; Zou, L. Downregulation of peroxiredoxin-1 by β-elemene enhances the radiosensitivity of lung adenocarcinoma xenografts. Oncol. Rep. 2015, 33, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Kwee, J.K. A paradoxical chemoresistance and tumor suppressive role of antioxidant in solid cancer cells: A strange case of Dr. Jekyll and Mr. Hyde. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Song, I.-S.; Kim, H.-K.; Jeong, S.-H.; Lee, S.-R.; Kim, N.; Rhee, B.D.; Ko, K.S.; Han, J. Mitochondrial peroxiredoxin III is a potential target for cancer therapy. Int. J. Mol. Sci. 2011, 12, 7163–7185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Su, Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett. 2009, 286, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Prosser, C.L.; Painter, E.E.; Lisco, H.; Brues, A.M.; Jacobson, L.O.; Swift, M.N. The Clinical Sequence of Physiological Effects of Ionizing Radiation in Animals. Radiology 1947, 49, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Popova, N.R.; Bruskov, V.I. Radioprotectors: History, Trends and Prospects. Biofizika 2015, 60, 801–811. [Google Scholar] [PubMed]
- Vasin, M.V. Comments on the mechanisms of action of radiation protective agents: Basis components and their polyvalence. Springerplus 2014, 3, 414. [Google Scholar] [CrossRef]
- Weiss, J.F.; Landauer, M.R. History and development of radiation-protective agents. Int. J. Radiat. Biol. 2009, 85, 539–573. [Google Scholar] [CrossRef]
- Gudkov, A.V.; Komarova, E.A. Radioprotection: Smart games with death. J. Clin. Investig. 2010, 120, 2270–2273. [Google Scholar] [CrossRef]
- Petkau, A. Role of superoxide dismutase in modification of radiation injury. Br. J. Cancer. Suppl. 1987, 8, 87–95. [Google Scholar]
- Petkau, A. Radiation protection by superoxide dismutase. Photochem. Photobiol. 1978, 28, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Epperly, M.W.; Melendez, J.A.; Zhang, X.; Nie, S.; Pearce, L.; Peterson, J.; Franicola, D.; Dixon, T.; Greenberger, B.A.; Komanduri, P.; et al. Mitochondrial targeting of a catalase transgene product by plasmid liposomes increases radioresistance in vitro and in vivo. Radiat. Res. 2009, 171, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Palutina, O.A.; Sharapov, M.G.; Temnov, A.A.; Novoselov, V.I. Nephroprotective Effect Exogenous Antioxidant Enzymes during Ischemia/Reperfusion-Induced Damage of Renal Tissue. Bull. Exp. Biol. Med. 2016, 160, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, S.V.; Peshenko, I.V.; Popov, V.I.; Novoselov, V.I.; Bystrova, M.F.; Evdokimov, V.J.; Kamzalov, S.S.; Merkulova, M.I.; Shuvaeva, T.M.; Lipkin, V.M.; et al. Localization of 28-kDa peroxiredoxin in rat epithelial tissues and its antioxidant properties. Cell Tissue Res. 1999, 298, 471–480. [Google Scholar] [CrossRef]
- Chuchalin, A.G.; Novoselov, V.I.; Shifrina, O.N.; Soodaeva, S.K.; Yanin, V.A.; Barishnikova, L.M. Peroxiredoxin VI in human respiratory system. Respir. Med. 2003, 97, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Gordeeva, A.E.; Temnov, A.A.; Charnagalov, A.A.; Sharapov, M.G.; Fesenko, E.E.; Novoselov, V.I. Protective Effect of Peroxiredoxin 6 in Ischemia/Reperfusion-Induced Damage of Small Intestine. Dig. Dis Sci. 2015, 60, 3610–3619. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Gordeeva, A.E.; Goncharov, R.G.; Tikhonova, I.V.; Ravin, V.K.; Temnov, A.A.; Fesenko, E.E.; Novoselov, V.I. The Effect of Exogenous Peroxiredoxin 6 on the State of Mesenteric Vessels and the Small Intestine in Ischemia–Reperfusion Injury. Biophysics 2017, 62, 998–1008. [Google Scholar] [CrossRef]
- Volkova, A.G.; Sharapov, M.G.; Ravin, V.K.; Gordeeva, A.E.; Karaduleva, E.V.; Mubarakshina, E.K.; Temnov, A.A.; Fesenko, E.E.; Novoselov, V.I. Effects of Different Antioxidant Enzymes on The Tracheal Epithelium Regeneration After Chemical Burn. Russ. Pulmonol. 2014, 84–90. [Google Scholar] [CrossRef]
- Sharapov, M.; Volkova, A.; Mubarakshina, E.; Novoselov, V.; Soodaeva, S.; Klimanov, I. Antioxidant systems in rat trachea upon thermal and chemical burns of upper airway. Eur. Respir. J. 2013, 42, P527. [Google Scholar]
- Sharapov, M.G.; Gudkov, S.V.; Gordeeva, A.E.; Karp, O.E.; Ivanov, V.E.; Shelkovskaya, O.V.; Bruskov, V.I.; Novoselov, V.I.; Fesenko, E.E. Peroxiredoxin 6 is a natural radioprotector. Dokl Biochem. Biophys. 2016, 467, 110–112. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Novoselov, V.I.; Fesenko, E.E.; Bruskov, V.I.; Gudkov, S.V. The role of peroxiredoxin 6 in neutralization of X-ray mediated oxidative stress: Effects on gene expression, preservation of radiosensitive tissues and postradiation survival of animals. Free Radic. Res. 2017, 51, 148–166. [Google Scholar] [CrossRef]
- Novoselov, V.I.; Ravin, V.K.; Sharapov, M.G.; Sofin, A.D.; Kukushkin, N.I.; Fesenko, E.E. Modified peroxiredoxins as prototypes of drugs with powerful antioxidant action. Biophysics 2011, 56. [Google Scholar] [CrossRef]
- Metcalf, D. Hematopoietic cytokines. Blood 2008, 111, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veltri, S.; Smith, J.W. Interleukin 1 trials in cancer patients: A review of the toxicity, antitumor and hematopoietic effects. Stem Cells 1996, 14, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.B.; Moulder, J.E.; Coleman, C.N.; Ang, K.K.; Anscher, M.S.; Barcellos-Hoff, M.H.; Dynan, W.S.; Fike, J.R.; Grdina, D.J.; Greenberger, J.S.; et al. Models for Evaluating Agents Intended for the Prophylaxis, Mitigation and Treatment of Radiation Injuries Report of an NCI Workshop, December 3–4, 2003. Radiat. Res. 2004, 162, 711–728. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Karp, O.E.; Bruskov, V.I. Long-lived protein radicals induced by X-ray irradiation are the source of reactive oxygen species in aqueous medium. Dokl Biochem. Biophys. 2010, 430, 1–4. [Google Scholar] [CrossRef]
- Karp, O.E.; Gudkov, S.V.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Bruskov, V.I. Genotoxic effect of long-lived protein radicals in vivo generated by X-ray irradiation. Dokl Biochem. Biophys. 2010, 434, 250–253. [Google Scholar] [CrossRef]
- Peskin, A.V.; Dickerhof, N.; Poynton, R.A.; Paton, L.N.; Pace, P.E.; Hampton, M.B.; Winterbourn, C.C. Hyperoxidation of peroxiredoxins 2 and 3: Rate constants for the reactions of the sulfenic acid of the peroxidatic cysteine. J. Biol. Chem. 2013, 288, 14170–14177. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A. Multiple functions of peroxiredoxins: Peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid. Redox Signal. 2011, 15, 781–794. [Google Scholar] [CrossRef]
- Wu, Y.; Feinstein, S.I.; Manevich, Y.; Chowdhury, I.; Pak, J.H.; Kazi, A.; Dodia, C.; Speicher, D.W.; Fisher, A.B. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A2 activity. Biochem. J. 2009, 419, 669–679. [Google Scholar] [CrossRef]
- Farooqui, A.A. Lipid Mediators in the Neural Cell Nucleus: Their Metabolism, Signaling, and Association with Neurological Disorders. Neuroscientist 2009, 15, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Wu, G.; Zhao, X.; Zhou, L.; Zhang, H.; Li, J.; Ma, L.; Zhang, Y. PRDX6 Protects ARPE-19 Cells from Oxidative Damage via PI3K/AKT Signaling. Cell. Physiol. Biochem. 2015, 36, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Wi, S.M.; Shin, D.; Chun, E.; Lee, K.-Y. Peroxiredoxin-6 Negatively Regulates Bactericidal Activity and NF-kB Activity by Interrupting TRAF6-ECSIT Complex. Front. Cell. Infect. Microbiol. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Bienert, G.P.; Møller, A.L.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Tornroth-Horsefield, S.; Hedfalk, K.; Fischer, G.; Lindkvist-Petersson, K.; Neutze, R. Structural insights into eukaryotic aquaporin regulation. FEBS J. 2010, 584, 2580–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, T. Close teamwork between Nrf2 and peroxiredoxins 1 and 6 for the regulation of prostaglandin D2 and E2 production in macrophages in acute inflammation. Free Radic. Biol. Med. 2015, 88, 189–198. [Google Scholar] [CrossRef]
- Baldwin, A.S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Investig. 2001, 107, 241–246. [Google Scholar] [CrossRef]
- Takada, Y.; Mukhopadhyay, A.; Kundu, G.C.; Mahabeleshwar, G.H.; Singh, S.; Aggarwal, B.B. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: Evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 2003, 278, 24233–24241. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.-S.; Yu, S.; Kong, A.-N. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef]
- Liu, G.-H.; Qu, J.; Shen, X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription factors NRF2 and NF-κB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhao, L.; Zhao, Q.; Zhao, Y.; Sun, Y.; Zhang, Y.; Miao, H.; You, Q.-D.; Hu, R.; Guo, Q.-L. NF-κB and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of inflammation-associated colorectal carcinogenesis. Cell. Death Dis. 2014, 5, e1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xia, T.; Yu, X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm. Res. 2015, 64, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.-K.; Bae, J.-S. Anti-inflammatory Effects of Baicalin, Baicalein, and Wogonin In Vitro and In Vivo. Inflammation 2015, 38, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Rithidech, K.N.; Reungpatthanaphong, P.; Honikel, L.; Rusek, A.; Simon, S.R. Dose-rate effects of protons on in vivo activation of nuclear factor-kappa B and cytokines in mouse bone marrow cells. Radiat. Environ. Biophys. 2010, 49, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Vénéreau, E.; Ceriotti, C.; Bianchi, M.E. DAMPs from Cell Death to New Life. Front. Immunol. 2015, 6, 422. [Google Scholar] [CrossRef]
- Riddell, J.R.; Bshara, W.; Moser, M.T.; Spernyak, J.A.; Foster, B.A.; Gollnick, S.O. Peroxiredoxin 1 controls prostate cancer growth through Toll-like receptor 4-dependent regulation of tumor vasculature. Cancer Res. 2011, 71, 1637–1646. [Google Scholar] [CrossRef]
- Shichita, T.; Hasegawa, E.; Kimura, A.; Morita, R.; Sakaguchi, R.; Takada, I.; Sekiya, T.; Ooboshi, H.; Kitazono, T.; Yanagawa, T.; et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 2012, 18, 911–917. [Google Scholar] [CrossRef]
- Whitaker, H.C.; Patel, D.; Howat, W.J.; Warren, A.Y.; Kay, J.D.; Sangan, T.; Marioni, J.C.; Mitchell, J.; Aldridge, S.; Luxton, H.J.; et al. Peroxiredoxin-3 is overexpressed in prostate cancer and promotes cancer cell survival by protecting cells from oxidative stress. Br. J. Cancer 2013, 109, 983–993. [Google Scholar] [CrossRef] [Green Version]
- Feldman, N.; Rotter-Maskowitz, A.; Okun, E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res. Rev. 2015, 24, 29–39. [Google Scholar] [CrossRef]
- Du, J.-R.; Kuang, X.; Wang, L.-F.; Yu, L.; Li, Y.-J.; Wang, Y.-N.; He, Q.; Chen, C.; Du, J.-R. Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: Involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic. Biol. Med. 2014, 71, 165–175. [Google Scholar] [CrossRef]
- Riddell, J.R.; Wang, X.-Y.; Minderman, H.; Gollnick, S.O. Peroxiredoxin 1 stimulates secretion of proinflammatory cytokines by binding to TLR4. J. Immunol. 2010, 184, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, C.E. The Nuclear Factor κB pathway: A link to the immune system in the radiation response. Cancer Lett. 2015, 368, 275–289. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Yokoo, T.; Kitamura, M. c-Jun/AP-1, but not NF-kappa B, is a mediator for oxidant-initiated apoptosis in glomerular mesangial cells. Biochem. Biophys. Res. Commun. 1997, 240, 496–501. [Google Scholar] [CrossRef]
- Turpaev, K.T. Role of transcription factor AP-1 in integration of cellular signalling systems. Mol. Biol. (Mosk) 2006, 40, 945–961. [Google Scholar] [CrossRef] [PubMed]
- Garces de los Fayos Alonso, I.; Liang, H.-C.; Turner, S.; Lagger, S.; Merkel, O.; Kenner, L. The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas. Cancers (Basel) 2018, 10, 93. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Moreno-Manzano, V.; Ishikawa, Y.; Lucio-Cazana, J.; Kitamura, M. Suppression of Apoptosis by All-trans-Retinoic Acid. J. Biol. Chem. 1999, 274, 20251–20258. [Google Scholar] [CrossRef] [Green Version]
- Yokoo, T.; Kitamura, M. Unexpected protection of glomerular mesangial cells from oxidant-triggered apoptosis by bioflavonoid quercetin. Am. J. Physiol. 1997, 273, F206-12. [Google Scholar] [CrossRef]
- Park, S. Polyphenol Compound as a Transcription Factor Inhibitor. Nutrients 2015, 7, 8987–9004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigoriev, P.A.; Sharapov, M.G.; Novoselov, V.I. Voltage-dependent cation-selective ion channels formed by peroxiredoxin 6 in a lipid bilayer. Biophysics 2015, 60, 696–699. [Google Scholar] [CrossRef]
- Chatterjee, S.; Feinstein, S.I.; Dodia, C.; Sorokina, E.; Lien, Y.C.; Nguyen, S.; Debolt, K.; Speicher, D.; Fisher, A.B. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J. Biol. Chem. 2011, 286, 11696–11706. [Google Scholar] [CrossRef] [PubMed]
- Manevich, Y.; Shuvaeva, T.; Dodia, C.; Kazi, A.; Feinstein, S.I.; Fisher, A.B. Binding of peroxiredoxin 6 to substrate determines differential phospholipid hydroperoxide peroxidase and phospholipase A2 activities. Arch. Biochem. Biophys. 2009, 485, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.B.; Vick, K.A.; Skoko, J.J.; Neumann, C.A. Peroxiredoxin involvement in the initiation and progression of human cancer. Antioxid. Redox Signal. 2017, 28, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Levanova, A.; Poranen, M.M. RNA Interference as a Prospective Tool for the Control of Human Viral Infections. Front. Microbiol. 2018, 9, 2151. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Liu, K.; Yang, X.; Song, M.; Wang, Y.; Bai, Y. Adenovirus-mediated transfer of siRNA against peroxiredoxin I enhances the radiosensitivity of human intestinal cancer. Biochem. Pharmacol. 2008, 75, 660–667. [Google Scholar] [CrossRef]
- Do Yo, Y.; Chung, Y.M.; Park, J.K.; Ahn, C.M.; Kim, S.K.; Kim, H.J. Synergistic effect of peroxiredoxin II antisense on cisplatin-induced cell death. Exp. Mol. Med. 2002, 34, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Guryev, E.L.; Volodina, N.O.; Shilyagina, N.Y.; Gudkov, S.V.; Balalaeva, I.V.; Volovetskiy, A.B.; Lyubeshkin, A.L.; Sen, A.V.; Ermilov, S.A.; Vodeneev, V.A.; et al. Radioactive (90Y) upconversion nanoparticles conjugated with recombinant targeted toxin for synergistic nanotheranostics of cancer. Proc. Natl. Acad. Sci. USA 2018, 115, 9690–9695. [Google Scholar] [CrossRef]
- Kłossowski, S.; Muchowicz, A.; Firczuk, M.; Świech, M.; Redzej, A.; Golab, J.; Ostaszewski, R.; Swiech, M.; Redzej, A.; Golab, J.; et al. Studies toward novel peptidomimetic inhibitors of thioredoxin-thioredoxin reductase system. J. Med. Chem. 2012, 55, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Trzeciecka, A.; Klossowski, S.; Bajor, M.; Zagozdzon, R.; Gaj, P.; Muchowicz, A.; Malinowska, A.; Czerwoniec, A.; Barankiewicz, J.; Domagala, A.; et al. Dimeric peroxiredoxins are druggable targets in human Burkitt lymphoma. Oncotarget 2016, 7, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- Graczyk-Jarzynka, A.; Zagozdzon, R.; Muchowicz, A.; Siernicka, M.; Juszczynski, P.; Firczuk, M. New insights into redox homeostasis as a therapeutic target in B-cell malignancies. Curr. Opin. Hematol. 2017, 24, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Jo, M.; Yun, H.-M.; Park, K.-R.; Park, M.H.; Lee, D.H.; Cho, S.H.; Yoo, H.-S.S.; Lee, Y.-M.; Jeong, H.S.; Kim, Y.; et al. Anti-cancer effect of thiacremonone through down regulation of peroxiredoxin 6. PLoS ONE 2014, 9, e91508. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Park, M.H.; Son, D.J.; Song, H.S.; Kim, J.H.; Ko, S.C.; Song, M.J.; Lee, W.H.; Yoon, J.H.; Ham, Y.W.; et al. Anti-cancer effect of snake venom toxin through down regulation of AP-1 mediated PRDX6 expression. Oncotarget 2015, 6, 22139–22151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudkov, S.V.; Guryev, E.L.; Gapeyev, A.B.; Sharapov, M.G.; Bunkin, N.F.; Shkirin, A.V.; Zabelina, T.S.; Glinushkin, A.P.; Sevost’yanov, M.A.; Belosludtsev, K.N.; et al. Unmodified hydrated C60 fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen species and protect mice against injuries caused by radiation-induced oxidative stress. Nanomedicine 2019, 15, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Vasin, M.V.; Semenov, L.F.; Suvorov, N.N.; Antipov, V.V.; Ushakov, I.B.; Ilyin, L.A.; Lapin, B.A. Protective effect and the therapeutic index of indralin in juvenile rhesus monkeys. J. Radiat. Res. 2014, 55, 1048–1055. [Google Scholar] [CrossRef] [Green Version]
- Vasin, M.V. Classification of radiation protective agents as a basis of modern radiation pharmacology. Radiat. Biol. Radioecol. 1999, 39, 212–222. [Google Scholar]
- Pacifici, F.; Della-Morte, D.; Capuani, B.; Pastore, D.; Bellia, A.; Sbraccia, P.; Di Daniele, N.; Lauro, R.; Lauro, D. Peroxiredoxin6, a Multitask Antioxidant Enzyme Involved in the Pathophysiology of Chronic Non-Communicable Diseases. Antioxid. Redox Signal. 2018, 30, 399–414. [Google Scholar] [CrossRef]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef]
- Nicolussi, A.; D’inzeo, S.; Capalbo, C.; Giannini, G.; Coppa, A. The role of peroxiredoxins in cancer. Mol. Clin. Oncol. 2017, 6, 139–153. [Google Scholar] [CrossRef] [Green Version]











| Type of Cancer | Mechanism of Action/Molecular Target | References |
|---|---|---|
| Brain | resistance to reactive oxygen species (ROS) | [77] |
| Lungs | resistance to ROS, activation of JAK2/STAT3, stimulation of metastasis, activation of iPLA2 activity, P38 activation via PI3K/Akt | [78,79,80] |
| Breast | resistance to ROS, stimulation of metastasis, stimulation of expression uPAR, Est-1, MMP-9, RhoC, TIMP-2 | [81,82] |
| Esophagus | resistance to ROS | [83] |
| Stomach | resistance to ROS, stimulation of metastasis, suppression of caspase-8 activation | [84] |
| Cervix | resistance to ROS, suppression of TRAIL activated caspase-10 | [85] |
| Liver | resistance to ROS | [86] |
| Ovaries | resistance to ROS, stimulation of metastasis | [87] |
| Bladder | resistance to ROS, stimulation of NF-kB | [51] |
| Prostate | resistance to ROS, stimulation of metastasis | [88] |
| Skin | resistance to ROS, stimulation of metastasis via activation of aiPLA2 activity | [89] |
| Type of Radioprotective Compounds | Mechanism of Action | Time of Medication | Tissue Specificity | DRF * |
|---|---|---|---|---|
| Sulfhydryl compounds | - antiradical; - donation of H-atom; - formation of mixed disulfides; -hypoxia (↓); -redox regulation (↓) | before irradiation | all tissues | 1.3–2.7 |
| Antioxidants | - antiradical; - redox regulation (↓); - hypoxia (↓); -immunity stimulation (↓) | before irradiation | all tissues | 1.1–1.3 |
| Inhibitors of ACE | -effects on the renin–angiotensin system; - inhibition of collagen synthesis ? (↓) | after irradiation | does not protect the gastrointestinal tract | <1.2 |
| Immunomodulators and cytokines | - stimulation of immunity; - cytokine production; - signaling cascades | before or after irradiation | mostly hematopoietic system | 1.1–1.4 |
| Prostaglandins | - tissue hormones - ? (↓) | before irradiation | hematopoietic system, gastrointestinal tract, hair follicles | <1.3 |
| Metal salts and metallothionein | - induction of metallothionein; - antiradical | before irradiation | mostly hematopoietic system | <1.2 |
| DNA_binding agents | - electron transfer; - compaction of chromatin (↓) | before irradiation | all tissues | <1.3 |
| Hypoxia_inducing compounds | - hypoxia; - redox regulation (↓) | before irradiation | all tissues | 1.2–1.5 |
| Selenium containing compounds | - stimulation of glutathione peroxidase activity; - antiradical; - redox regulation (↓) | before or after irradiation | mostly gastrointestinal tract | <1.3 |
| Nucleic acids and their derivatives | - antiradical; - effect on repair systems; - signaling cascades; - ? (↓) | before or after irradiation | all tissues | 1.1–1.4 |
| Fullerenes | - antiradical; - membrane protection; - ? (↓) | before exposure | all tissues | <1.3 |
| Adsorbents | - binding of radionuclides; - acceleration of radionuclide excretion | after irradiation | gastrointestinal tract | ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharapov, M.G.; Novoselov, V.I.; Gudkov, S.V. Radioprotective Role of Peroxiredoxin 6. Antioxidants 2019, 8, 15. https://doi.org/10.3390/antiox8010015
Sharapov MG, Novoselov VI, Gudkov SV. Radioprotective Role of Peroxiredoxin 6. Antioxidants. 2019; 8(1):15. https://doi.org/10.3390/antiox8010015
Chicago/Turabian StyleSharapov, Mars G., Vladimir I. Novoselov, and Sergey V. Gudkov. 2019. "Radioprotective Role of Peroxiredoxin 6" Antioxidants 8, no. 1: 15. https://doi.org/10.3390/antiox8010015