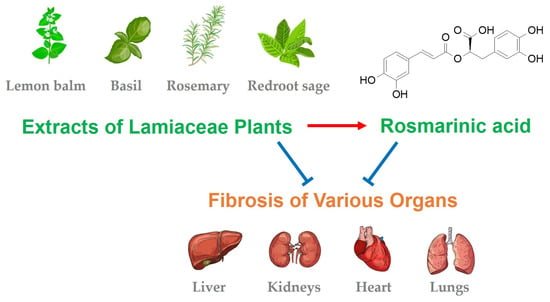
Therapeutic Potential and Mechanisms of Rosmarinic Acid and the Extracts of Lamiaceae Plants for the Treatment of Fibrosis of Various Organs
Abstract
:1. Introduction
2. Common Mediators of Fibrosis
2.1. TGF-β
2.2. PDGF
2.3. CTGF
2.4. Wnt
2.5. HSPs
2.6. ROS
3. Methods: Study Search and Selection
4. Ex Vivo Fibrosis Model Studies
5. In Vivo Fibrosis Model Studies: Antifibrotic Effects of RA and the Plant Extracts Containing RA
5.1. Liver Fibrosis
5.2. Kidney Fibrosis
5.3. Heart Fibrosis
5.4. Lung Fibrosis
5.5. Post-Surgical Abdominal Adhesion
5.6. Fibrosis in the Salivary Glands
5.7. Skin Wounds
5.8. Pterygium in the Eyes
5.9. Fibrosis of Autologous Fat Grafts
6. Lamiaceae Plants with Antifibrotic Effects
7. Key Mediators of the Antifibrotic Effects of RA
7.1. PPARγ
7.2. AMPK
7.3. NRF2
7.4. NF-κB
8. Future Perspectives
- To verify key mediators of RA effects as therapeutic targets for fibrotic diseases.
- To compare RA with other competing substances in terms of efficacy and safety.
- To discover new drug candidates using RA as a lead compound.
- To develop formulations to enhance the bioavailability and targeted delivery of RA.
- To evaluate the safety and therapeutic efficacy of RA through clinical trials.
- To expand the industrial and medical applications of RA.
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| ACE | Angiotensin-converting enzyme |
| AGEs | Advanced glycation end-products |
| ALT | Alanine aminotransferase |
| AMPK | 5′ AMP-activated protein kinase |
| Akt | Protein kinase B (PKB) |
| AP-1 | Activator protein 1 |
| Apaf-1 | Apoptotic protease activating factor-1 |
| ARE | Antioxidant response element |
| AST | Aspartate aminotransferase |
| AT1R | Angiotensin type 1 receptor |
| Bad | Bcl-2-associated death protein |
| BALF | Bronchoalveolar lavage fluid |
| Bcl 2 | B cell lymphoma/leukemia 2 |
| BDL | Bile duct ligation |
| BUN | Blood urea nitrogen |
| CAMK | Calcium/calmodulin-dependent protein kinase |
| COL | Collagen |
| CTGF | Connective tissue growth factor |
| DKK1 | Dickkopf-1 |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| FAS | Fatty acid synthase |
| FZHY | Fuzheng Huayu recipe |
| G6PDH | Glucose-6-phosphate dehydrogenase |
| GCLc | Catalytic subunits of glutamate cysteine ligase |
| GPX | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GST | Glutathione S-transferase |
| HO | Heme oxygenase |
| HSCs | Hepatic stellate cells |
| HSF | Heat shock factor |
| HSPs | Heat shock proteins |
| IFN-γ | Interferon gamma |
| Ig | Immunoglobulin |
| IKK | IκB kinase |
| IL | Interleukin |
| JNK | c-Jun N-terminal kinase |
| KEAP | Kelch-like ECH-associated protein |
| LAD | Left anterior descending coronary artery |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MCD | Methionine- and choline-deficient |
| MDA | Malondialdehyde |
| MDCKs | Madin–Darby canine kidney cells |
| MI | Myocardial infarction |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear factor kappa B |
| NOS | Nitric oxide synthase |
| NOX | NADPH oxidase |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| PCLS | Precision-cut liver slices |
| PCOL | Procollagen |
| PDGF | Platelet-derived growth factor |
| PDGFR | PDGF receptor |
| PGC | PPARγ coactivator |
| PI3K | Phosphoinositide 3-kinase |
| PKC | Protein kinase C |
| PLA | Phospholipase A |
| PLC | Phospholipase C |
| PPAR | Peroxisomal proliferator-activated receptor |
| RA | Rosmarinic acid |
| ROS | Reactive oxygen species |
| RXR | Retinoid X receptor |
| SD | Sprague Dawley |
| shRNA | Short hairpin RNA |
| SMA | Smooth muscle actin |
| SMAD | Small mothers against decapentaplegic |
| SOD | Superoxide dismutase |
| SREBP | Sterol regulatory element-binding protein |
| STAT | Signal transducers and activators of transcription |
| TGF | Transforming growth factor |
| TGF-βR | TGF-β receptor |
| TIMP | Tissue inhibitor of metalloproteinase |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| TNFR | TNF receptor |
| TrCP | Transducin repeat-containing protein |
| UUO | Unilateral ureteral obstruction |
| WHW | Wen-pi-tang-Hab-Wu-ling-san |
References
- Lee, S.I.; Kim, H.J.; Baek, M.C.; Park, K.M.; Park, Y.; Yoon, C.H.; Boo, Y.C. Wen-pi-tang-Hab-Wu-ling-san, an oriental herbal prescription, attenuates epithelial-mesenchymal transdifferentiation stimulated by TGF-beta1 in kidney cells. Phytother. Res. 2007, 21, 548–553. [Google Scholar] [CrossRef]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef] [PubMed]
- Lurje, I.; Gaisa, N.T.; Weiskirchen, R.; Tacke, F. Mechanisms of organ fibrosis: Emerging concepts and implications for novel treatment strategies. Mol. Asp. Med. 2023, 92, 101191. [Google Scholar] [CrossRef] [PubMed]
- Gyorfi, A.H.; Matei, A.E.; Distler, J.H.W. Targeting TGF-beta signaling for the treatment of fibrosis. Matrix Biol. 2018, 68–69, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.W.; Gyorfi, A.H.; Ramanujam, M.; Whitfield, M.L.; Konigshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021, 9, 730176. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Yanagihara, T.; Kolb, M.R.J. Therapeutic targets and early stage clinical trials for pulmonary fibrosis. Expert Opin. Investig. Drugs 2019, 28, 19–28. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Valdivielso, J.M. New therapeutic targets in chronic kidney disease progression and renal fibrosis. Expert Opin. Ther. Targets 2020, 24, 655–670. [Google Scholar] [CrossRef]
- Park, S.; Nguyen, N.B.; Pezhouman, A.; Ardehali, R. Cardiac fibrosis: Potential therapeutic targets. Transl. Res. 2019, 209, 121–137. [Google Scholar] [CrossRef]
- Latief, U.; Ahmad, R. Herbal remedies for liver fibrosis: A review on the mode of action of fifty herbs. J. Tradit. Complement. Med. 2018, 8, 352–360. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Wang, H.; Lu, Y. Research and application of herbal medicine in the treatment of chronic kidney disease since the 21st century: A visualized bibliometric analysis. Front. Pharmacol. 2022, 13, 971113. [Google Scholar] [CrossRef]
- Wu, X.; Huang, J.; Wang, J.; Xu, Y.; Yang, X.; Sun, M.; Shi, J. Multi-Pharmaceutical Activities of Chinese Herbal Polysaccharides in the Treatment of Pulmonary Fibrosis: Concept and Future Prospects. Front. Pharmacol. 2021, 12, 707491. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, T.; Feng, D.; Li, R.; Zhang, C. Polyphenols from Chinese Herbal Medicine: Molecular Mechanisms and Therapeutic Targets in Pulmonary Fibrosis. Am. J. Chin. Med. 2022, 50, 1063–1094. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxid. Med. Cell Longev. 2018, 2018, 8394818. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Qiao, Y.; Zhao, Y.; Chen, X.; Li, J.; Zhang, H.; Lan, Q.; Yang, B. Natural products: Potential drugs for the treatment of renal fibrosis. Chin. Med. 2022, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A. Importance of dietary hydroxycinnamic acids in the therapy of liver fibrosis. Orv. Hetil. 2012, 153, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Elufioye, T.O.; Habtemariam, S. Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action. Biomed. Pharmacother. 2019, 112, 108600. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Dahchour, A. Anxiolytic and antidepressive potentials of rosmarinic acid: A review with a focus on antioxidant and anti-inflammatory effects. Pharmacol. Res. 2022, 184, 106421. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, L.; Jin, D.; Xin, Y.; Tian, L.; Wang, T.; Zhao, D.; Wang, Z.; Wang, J. Rosmarinic Acid and Related Dietary Supplements: Potential Applications in the Prevention and Treatment of Cancer. Biomolecules 2022, 12, 1410. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.K.; Anwar, S.; Hasan, G.M.; Shamsi, A.; Islam, A.; Parvez, S.; Hassan, M.I. Comprehensive Insights into Biological Roles of Rosmarinic Acid: Implications in Diabetes, Cancer and Neurodegenerative Diseases. Nutrients 2023, 15, 4297. [Google Scholar] [CrossRef] [PubMed]
- Budi, E.H.; Schaub, J.R.; Decaris, M.; Turner, S.; Derynck, R. TGF-β as a driver of fibrosis: Physiological roles and therapeutic opportunities. J. Pathol. 2021, 254, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Peng, D.; Zhang, Y.; Shi, H. TGF-beta signal transduction: Biology, function and therapy for diseases. Mol. Biomed. 2022, 3, 45. [Google Scholar] [CrossRef]
- Klinkhammer, B.M.; Floege, J.; Boor, P. PDGF in organ fibrosis. Mol. Asp. Med. 2018, 62, 44–62. [Google Scholar] [CrossRef]
- Zou, X.; Tang, X.Y.; Qu, Z.Y.; Sun, Z.W.; Ji, C.F.; Li, Y.J.; Guo, S.D. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: A review. Int. J. Biol. Macromol. 2022, 202, 539–557. [Google Scholar] [CrossRef]
- Reyhani, V.; Tsioumpekou, M.; van Wieringen, T.; Rask, L.; Lennartsson, J.; Rubin, K. PDGF-BB enhances collagen gel contraction through a PI3K-PLCγ-PKC-cofilin pathway. Sci. Rep. 2017, 7, 8924. [Google Scholar] [CrossRef]
- Wang, J.; You, J.; Gong, D.; Xu, Y.; Yang, B.; Jiang, C. PDGF-BB induces conversion, proliferation, migration, and collagen synthesis of oral mucosal fibroblasts through PDGFR-beta/PI3K/ AKT signaling pathway. Cancer Biomark. 2021, 30, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Takamura, N.; Renaud, L.; da Silveira, W.A.; Feghali-Bostwick, C. PDGF Promotes Dermal Fibroblast Activation via a Novel Mechanism Mediated by Signaling Through MCHR1. Front. Immunol. 2021, 12, 745308. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Rathnakar, B.H.; Kwon, H.R.; Sakashita, H.; Kim, J.H.; Rackley, A.; Tomasek, J.J.; Berry, W.L.; Olson, L.E. Temporal control of PDGFRalpha regulates the fibroblast-to-myofibroblast transition in wound healing. Cell Rep. 2022, 40, 111192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Kong, D.; Chen, L.; Zhang, X.; Lian, N.; Zhu, X.; Lu, Y.; Zheng, S. Peroxisome proliferator-activated receptor-gamma interrupts angiogenic signal transduction by transrepression of platelet-derived growth factor-beta receptor in hepatic stellate cells. J. Cell Sci. 2014, 127, 305–314. [Google Scholar] [PubMed]
- Kikuchi, A.; Pradhan-Sundd, T.; Singh, S.; Nagarajan, S.; Loizos, N.; Monga, S.P. Platelet-Derived Growth Factor Receptor alpha Contributes to Human Hepatic Stellate Cell Proliferation and Migration. Am. J. Pathol. 2017, 187, 2273–2287. [Google Scholar] [CrossRef] [PubMed]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012, 5, S24. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, N.; Chu, H.Y.; Yu, Y.; Zhang, Z.K.; Zhang, G.; Zhang, B.T. Connective Tissue Growth Factor: From Molecular Understandings to Drug Discovery. Front. Cell Dev. Biol. 2020, 8, 593269. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Peng, D.; Lan, T.; Wei, Y.; Wei, X. Multifunctional regulatory protein connective tissue growth factor (CTGF): A potential therapeutic target for diverse diseases. Acta Pharm. Sin. B 2022, 12, 1740–1760. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, M.; Chen, J.; Gai, J.; Huang, J.; Zhou, Y.; Wan, Y.; Tu, C. Identification and Characterization of a Novel Nanobody Against Human CTGF to Reveal Its Antifibrotic Effect in an in vitro Model of Liver Fibrosis. Int. J. Nanomed. 2023, 18, 5407–5422. [Google Scholar] [CrossRef]
- Trampuz, S.R.; van Riet, S.; Nordling, A.; Ingelman-Sundberg, M. The Role of CTGF in Liver Fibrosis Induced in 3D Human Liver Spheroids. Cells 2023, 12, 302. [Google Scholar] [CrossRef]
- Barbe, M.F.; Hilliard, B.A.; Amin, M.; Harris, M.Y.; Hobson, L.J.; Cruz, G.E.; Popoff, S.N. Blocking CTGF/CCN2 reduces established skeletal muscle fibrosis in a rat model of overuse injury. FASEB J. 2020, 34, 6554–6569. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Xiao, Q.; Xiao, J.N.; Niu, C.X.; Li, Y.Y.; Zhang, X.J.; Zhou, Z.W.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.G.; Luo, Y.; He, D.L.; Li, X.; Zhang, L.L.; Peng, T.; Li, M.C.; Lin, Y.H. Role of Wnt/β-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1α. Int. J. Urol. 2007, 14, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Lyros, O.; Rafiee, P.; Nie, L.H.; Medda, R.; Jovanovic, N.; Schmidt, J.; Mackinnon, A.; Venu, N.; Shaker, R. Dickkopf-1, the Wnt antagonist, is induced by acidic pH and mediates epithelial cellular senescence in human reflux esophagitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G557–G574. [Google Scholar] [CrossRef]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Huang, W.; Ge, X. The role of heat shock proteins in the regulation of fibrotic diseases. Biomed. Pharmacother. 2021, 135, 111067. [Google Scholar] [CrossRef] [PubMed]
- Bunch, H.; Calderwood, S.K. Role of Heat Shock Factors in Stress-Induced Transcription: An Update. Methods Mol. Biol. 2023, 2693, 25–38. [Google Scholar] [PubMed]
- Kmiecik, S.W.; Mayer, M.P. Molecular mechanisms of heat shock factor 1 regulation. Trends Biochem. Sci. 2022, 47, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, T.; Huang, J.; Ma, K.; Xu, L.; Wang, H.; Fan, X.; Tao, R.; Ai, G.; Ning, Q. Expression of heat shock protein 47, transforming growth factor-beta 1, and connective tissue growth factor in liver tissue of patients with Schistosoma japonicum-induced hepatic fibrosis. Parasitology 2015, 142, 341–351. [Google Scholar] [CrossRef]
- Lonsmann, I.; Gudmann, N.S.; Manon-Jensen, T.; Thiele, M.; Moreno, Y.M.; Langholm, L.L.; Nielsen, M.J.; Detlefsen, S.; Karsdal, M.A.; Krag, A.A.; et al. Serologically assessed heat shock protein 47 is related to fibrosis stage in early compensated alcohol-related liver disease. Clin. Biochem. 2022, 104, 36–43. [Google Scholar] [CrossRef]
- Bellaye, P.S.; Burgy, O.; Bonniaud, P.; Kolb, M. HSP47: A potential target for fibrotic diseases and implications for therapy. Expert Opin. Ther. Targets 2021, 25, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, A.; Wilson, N.A.; Djamali, A. Heat shock protein 27 (HSP27): Biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair. 2012, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Park, A.M.; Kanai, K.; Itoh, T.; Sato, T.; Tsukui, T.; Inagaki, Y.; Selman, M.; Matsushima, K.; Yoshie, O. Heat Shock Protein 27 Plays a Pivotal Role in Myofibroblast Differentiation and in the Development of Bleomycin-Induced Pulmonary Fibrosis. PLoS ONE 2016, 11, e0148998. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Kietzmann, T. Reactive oxygen species and fibrosis: Further evidence of a significant liaison. Cell Tissue Res. 2016, 365, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.Q. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef]
- Saso, L.; Reza, A.; Ng, E.; Nguyen, K.; Lin, S.; Zhang, P.Z.; Fantozzi, P.J.; Armagan, G.; Romeo, U.; Cirillo, N. A Comprehensive Analysis of the Role of Oxidative Stress in the Pathogenesis and Chemoprevention of Oral Submucous Fibrosis. Antioxidants 2022, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.S.; Wang, S.; Liang, J.W.; Li, T.; Zhou, L.; Zhan, Z.L.; Wan, X.F.; Kang, C.Z.; Guo, L.P. Cloning and functional analysis of caffeic acid and rosmarinic acid glycosyltransferases from Arnebia euchroma. Zhongguo Zhong Yao Za Zhi 2021, 46, 86–93. [Google Scholar]
- Westra, I.M.; Oosterhuis, D.; Groothuis, G.M.; Olinga, P. Precision-cut liver slices as a model for the early onset of liver fibrosis to test antifibrotic drugs. Toxicol. Appl. Pharmacol. 2014, 274, 328–338. [Google Scholar] [CrossRef]
- Westra, I.M.; Oosterhuis, D.; Groothuis, G.M.; Olinga, P. The effect of antifibrotic drugs in rat precision-cut fibrotic liver slices. PLoS ONE 2014, 9, e95462. [Google Scholar] [CrossRef]
- Westra, I.M.; Mutsaers, H.A.; Luangmonkong, T.; Hadi, M.; Oosterhuis, D.; de Jong, K.P.; Groothuis, G.M.; Olinga, P. Human precision-cut liver slices as a model to test antifibrotic drugs in the early onset of liver fibrosis. Toxicol. Vitr. 2016, 35, 77–85. [Google Scholar] [CrossRef]
- Iswandana, R.; Pham, B.T.; van Haaften, W.T.; Luangmonkong, T.; Oosterhuis, D.; Mutsaers, H.A.; Olinga, P. Organ- and species-specific biological activity of rosmarinic acid. Toxicol. Vitr. 2016, 32, 261–268. [Google Scholar] [CrossRef]
- Li, G.S.; Jiang, W.L.; Tian, J.W.; Qu, G.W.; Zhu, H.B.; Fu, F.H. In vitro and in vivo antifibrotic effects of rosmarinic acid on experimental liver fibrosis. Phytomedicine 2010, 17, 282–288. [Google Scholar] [CrossRef]
- Yang, M.D.; Chiang, Y.M.; Higashiyama, R.; Asahina, K.; Mann, D.A.; Mann, J.; Wang, C.C.; Tsukamoto, H. Rosmarinic acid and baicalin epigenetically derepress peroxisomal proliferator-activated receptor gamma in hepatic stellate cells for their antifibrotic effect. Hepatology 2012, 55, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Lin, S.Y.; Chen, W.Y.; Liao, S.L.; Wu, C.C.; Pan, P.H.; Chou, S.T.; Chen, C.J. Glechoma hederacea extracts attenuate cholestatic liver injury in a bile duct-ligated rat model. J. Ethnopharmacol. 2017, 204, 58–66. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, Y.Y.; Chen, W.Y.; Liao, S.L.; Chou, S.T.; Yang, C.P.; Chen, C.J. Hepatoprotective activities of rosmarinic acid against extrahepatic cholestasis in rats. Food Chem. Toxicol. 2017, 108, 214–223. [Google Scholar] [CrossRef] [PubMed]
- El-Lakkany, N.M.; El-Maadawy, W.H.; Seif El-Din, S.H.; Hammam, O.A.; Mohamed, S.H.; Ezzat, S.M.; Safar, M.M.; Saleh, S. Rosmarinic acid attenuates hepatic fibrogenesis via suppression of hepatic stellate cell activation/proliferation and induction of apoptosis. Asian Pac. J. Trop. Med. 2017, 10, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yoo, G.; Randy, A.; Son, Y.J.; Hong, C.R.; Kim, S.M.; Nho, C.W. Lemon Balm and Its Constituent, Rosmarinic Acid, Alleviate Liver Damage in an Animal Model of Nonalcoholic Steatohepatitis. Nutrients 2020, 12, 1166. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Kong, W.; Liu, Z.; Wang, S.; Zhao, P.; Liang, K.; Niu, Y.; Yang, W.; Xiang, C.; Hu, X.; et al. Advanced glycation end-products as mediators of the aberrant crosslinking of extracellular matrix in scarred liver tissue. Nat. Biomed. Eng. 2023, 7, 1437–1454. [Google Scholar] [CrossRef] [PubMed]
- Seok, Y.M.; Kim, J.; Choi, K.C.; Yoon, C.H.; Boo, Y.C.; Park, Y.; Park, K.M. Wen-pi-tang-Hab-Wu-ling-san attenuates kidney ischemia/reperfusion injury in mice A role for antioxidant enzymes and heat-shock proteins. J. Ethnopharmacol. 2007, 111, 333. [Google Scholar] [CrossRef]
- Seok, Y.M.; Kim, J.; Park, M.J.; Boo, Y.C.; Park, Y.K.; Park, K.M. Wen-pi-tang-Hab-Wu-ling-san attenuates kidney fibrosis induced by ischemia/reperfusion in mice. Phytother. Res. 2008, 22, 1057. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.J.; Kim, J.; Park, Y.K.; Yoon, Y.R.; Park, K.M. Wen-pi-tang-Hab-Wu-ling-san reduces ureteral obstructive renal fibrosis by the reduction of oxidative stress, inflammation, and TGF-beta/Smad2/3 signaling. Food Chem. Toxicol. 2010, 48, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Kim, Y.J.; Seo, C.S.; Kim, H.T.; Park, S.R.; Lee, M.Y.; Jung, J.Y. Elsholtzia ciliata (Thunb.) Hylander attenuates renal inflammation and interstitial fibrosis via regulation of TGF-ss and Smad3 expression on unilateral ureteral obstruction rat model. Phytomedicine 2016, 23, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Tsai, J.P.; Ting, Y.H.; Hung, T.W.; Chao, W.W. Rosmarinic acid ameliorates renal interstitial fibrosis by inhibiting the phosphorylated-AKT mediated epithelial-mesenchymal transition in vitro and in vivo. Food Funct. 2022, 13, 4641–4652. [Google Scholar] [CrossRef] [PubMed]
- Joardar, S.; Dewanjee, S.; Bhowmick, S.; Dua, T.K.; Das, S.; Saha, A.; De Feo, V. Rosmarinic Acid Attenuates Cadmium-Induced Nephrotoxicity via Inhibition of Oxidative Stress, Apoptosis, Inflammation and Fibrosis. Int. J. Mol. Sci. 2019, 20, 2027. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, J.; Wang, D.; Zhou, F.; Cai, X.; Lu, W.; Hu, C.; Gu, Z.; Qian, S.; Guan, X.; et al. The aqueous extract of Lycopus lucidus Turcz ameliorates streptozotocin-induced diabetic renal damage via inhibiting TGF-beta1 signaling pathway. Phytomedicine 2013, 20, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, J.; Wang, S.; Liu, Y.; Long, K.; Sun, T.; Zhi, W.; Yang, Y.; Zhang, H.; Zhao, Y.; et al. Guanxining injection alleviates fibrosis in heart failure mice and regulates SLC7A11/GPX4 axis. J. Ethnopharmacol. 2023, 310, 116367. [Google Scholar] [CrossRef]
- Fathiazad, F.; Matlobi, A.; Khorrami, A.; Hamedeyazdan, S.; Soraya, H.; Hammami, M.; Maleki-Dizaji, N.; Garjani, A. Phytochemical screening and evaluation of cardioprotective activity of ethanolic extract of Ocimum basilicum L. (basil) against isoproterenol induced myocardial infarction in rats. Daru 2012, 20, 87. [Google Scholar] [CrossRef]
- Wei, J.; Leng, L.; Sui, Y.; Song, S.; Owusu, F.B.; Li, X.; Cao, Y.; Li, P.; Wang, H.; Li, R.; et al. Phenolic acids from Prunella vulgaris alleviate cardiac remodeling following myocardial infarction partially by suppressing NLRP3 activation. Phytother. Res. 2024, 38, 384–399. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Xu, Y.; Li, C.; Meng, X.; Fu, F. Protective Effect of RA on Myocardial Infarction-Induced Cardiac Fibrosis via AT1R/p38 MAPK Pathway Signaling and Modulation of the ACE2/ACE Ratio. J. Agric. Food Chem. 2016, 64, 6716–6722. [Google Scholar] [CrossRef]
- Zhang, L.; Bei, Z.; Li, T.; Qian, Z. An injectable conductive hydrogel with dual responsive release of rosmarinic acid improves cardiac function and promotes repair after myocardial infarction. Bioact. Mater. 2023, 29, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, Z.G.; Yuan, Y.P.; Xu, S.C.; Wei, W.Y.; Song, P.; Kong, C.Y.; Deng, W.; Tang, Q.Z. Rosmarinic acid attenuates cardiac fibrosis following long-term pressure overload via AMPKalpha/Smad3 signaling. Cell Death Dis. 2018, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Rahbardar, M.G.; Eisvand, F.; Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. In Vivo and In Vitro Protective Effects of Rosmarinic Acid against Doxorubicin-Induced Cardiotoxicity. Nutr. Cancer 2022, 74, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Ben Ali, R.; Gasmi, K.; Mlika, M.; Fazaa, S.; Ksouri, R.; Serairi, R.; Jameleddine, S.; Shlyonsky, V. Prophylactic and curative effect of rosemary leaves extract in a bleomycin model of pulmonary fibrosis. Pharm. Biol. 2017, 55, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Mies, F.; Ben Ali, R.; Mlika, M.; Jameleddine, S.; Mc Entee, K.; Shlyonsky, V. Rosmarinic acid potentiates carnosic acid induced apoptosis in lung fibroblasts. PLoS ONE 2017, 12, e0184368. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, S.; Liu, C.; Hu, K.; Xu, M.; Wang, R. Rosmarinic Acid Prevents Radiation-Induced Pulmonary Fibrosis through Attenuation of ROS/MYPT1/TGFbeta1 Signaling Via miR-19b-3p. Dose Response 2020, 18, 1559325820968413. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, L.; Zhang, X.; Long, Y.; Zou, F.; Yan, C.; Zou, W. Protective effects and active ingredients of Salvia miltiorrhiza Bunge extracts on airway responsiveness, inflammation and remodeling in mice with ovalbumin-induced allergic asthma. Phytomedicine 2019, 52, 168–177. [Google Scholar] [CrossRef]
- Shakeri, F.; Eftekhar, N.; Roshan, N.M.; Rezaee, R.; Moghimi, A.; Boskabady, M.H. Rosmarinic acid affects immunological and inflammatory mediator levels and restores lung pathological features in asthmatic rats. Allergol. Immunopathol. 2019, 47, 16–23. [Google Scholar] [CrossRef]
- Kakanezhadi, A.; Rezaei, M.; Raisi, A.; Dezfoulian, O.; Davoodi, F.; Ahmadvand, H. Rosmarinic acid prevents post-operative abdominal adhesions in a rat model. Sci. Rep. 2022, 12, 18593. [Google Scholar] [CrossRef]
- Peng, H.H.; Chen, Y.M.; Lee, C.I.; Lee, M.W. Synthesis of a disulfide cross-linked polygalacturonic acid hydrogel for biomedical applications. J. Mater. Sci. Mater. Med. 2013, 24, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, C.; Ma, S.; Gao, Y.; Wang, R. Protective Effect and Mechanism of Action of Rosmarinic Acid on Radiation-Induced Parotid Gland Injury in Rats. Dose Response 2020, 18, 1559325820907782. [Google Scholar] [CrossRef] [PubMed]
- Kuba, M.C.; Turkoglu, A.; Oguz, A.; Tuncer, M.C.; Kaya, S.; Basol, O.; Bilge, H.; Tatli, F. Comparison of local rosmarinic acid and topical dexpanthenol applications on wound healing in a rat experimental wound model. Folia Morphol. 2021, 80, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xu, Q.; Wei, X.; Ma, Q.; Li, D.; Zhao, J. Rosmarinic Acid-Grafted Dextran/Gelatin Hydrogel as a Wound Dressing with Improved Properties: Strong Tissue Adhesion, Antibacterial, Antioxidant and Anti-Inflammatory. Molecules 2023, 28, 4034. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Poelstra, K. Hepatic Stellate Cell Targeting Using Peptide-Modified Biologicals. Methods Mol. Biol. 2023, 2669, 269–284. [Google Scholar] [PubMed]
- Miao, C.G.; Yang, Y.Y.; He, X.; Huang, C.; Huang, Y.; Zhang, L.; Lv, X.W.; Jin, Y.; Li, J. Wnt signaling in liver fibrosis: Progress, challenges and potential directions. Biochimie 2013, 95, 2326–2335. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Wang, J.; Zhu, T.J.; Shen, Y.; Tang, X.S.; Fang, L.; Xu, Y.Z. Cross-Talking Between PPAR and WNT Signaling and its Regulation in Mesenchymal Stem Cell Differentiation. Curr. Stem Cell Res. Ther. 2016, 11, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Wang, Y.L.; Feng, X.B.; Song, X.D.; Liu, W.B. Rosmarinic acid inhibits proliferation and induces apoptosis of hepatic stellate cells. Biol. Pharm. Bull. 2011, 34, 343–348. [Google Scholar] [CrossRef]
- De Smet, V.; Eysackers, N.; Merens, V.; Kazemzadeh Dastjerd, M.; Halder, G.; Verhulst, S.; Mannaerts, I.; van Grunsven, L.A. Initiation of hepatic stellate cell activation extends into chronic liver disease. Cell Death Dis. 2021, 12, 1110. [Google Scholar] [CrossRef]
- Lu, C.; Zou, Y.; Liu, Y.; Niu, Y. Rosmarinic acid counteracts activation of hepatic stellate cells via inhibiting the ROS-dependent MMP-2 activity: Involvement of Nrf2 antioxidant system. Toxicol. Appl. Pharmacol. 2017, 318, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shen, D.P.; Wang, Q.L.; Tao, Y.Y.; Liu, C.H. Investigation of the absorbed and metabolized components of Danshen from Fuzheng Huayu recipe and study on the anti-hepatic fibrosis effects of these components. J. Ethnopharmacol. 2013, 148, 691–700. [Google Scholar] [CrossRef]
- Yang, T.; Liu, S.; Wang, C.H.; Tao, Y.Y.; Zhou, H.; Liu, C.H. Comparative pharmacokinetic and tissue distribution profiles of four major bioactive components in normal and hepatic fibrosis rats after oral administration of Fuzheng Huayu recipe. J. Pharm. Biomed. Anal. 2015, 114, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Yoon, C.H.; Kim, Y.H.; Boo, Y.C.; Park, K.M.; Park, Y.K. Wen-Pi-Tang-Hab-Wu-Ling-San extract inhibits the release of inflammatory mediators from LPS-stimulated mouse macrophages. J. Ethnopharmacol. 2007, 114, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Jung, J.K.; Ramalingam, M.; Yoon, C.H.; Bae, H.S.; Park, Y.K. Anti-diabetic effect of Wen-pi-tang-Hab-Wu-ling-san extract in streptozotocin-induced diabetic rats. Indian J. Pharmacol. 2012, 44, 97–102. [Google Scholar] [PubMed]
- Su, Z.; Wang, X.; Gao, X.; Liu, Y.; Pan, C.; Hu, H.; Beyer, R.P.; Shi, M.; Zhou, J.; Zhang, J.; et al. Excessive activation of the alternative complement pathway in autosomal dominant polycystic kidney disease. J. Intern. Med. 2014, 276, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Ma, H.; Zou, J.; Wang, W.; Zhu, L.; Deng, H.; Meng, M.; Tan, S.; Zhang, H.; Xiao, X.; et al. Metformin protects against ischaemic myocardial injury by alleviating autophagy-ROS-NLRP3-mediated inflammatory response in macrophages. J. Mol. Cell Cardiol. 2020, 145, 1–13. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.X.; Ma, Z.G.; Wu, H.M.; Xu, S.C.; Song, P.; Kong, C.Y.; Yuan, Y.P.; Deng, W.; Tang, Q.Z. Rosmarinic acid alleviates cardiomyocyte apoptosis via cardiac fibroblast in doxorubicin-induced cardiotoxicity. Int. J. Biol. Sci. 2019, 15, 556–567. [Google Scholar] [CrossRef]
- Bahri, S.; Ali, R.B.; Abdennabi, R.; Nahdi, A.; Mlika, M.; Jameleddine, S. Industrial Elimination of Essential Oils from Rosmarinus officinalis: In Support of the Synergic Antifibrotic Effect of Rosmarinic and Carnosic Acids in Bleomycin Model of Lung Fibrosis. Nutr. Cancer 2021, 73, 2376–2387. [Google Scholar] [CrossRef]
- Tang, J.; Xiang, Z.; Bernards, M.T.; Chen, S. Peritoneal adhesions: Occurrence, prevention and experimental models. Acta Biomater. 2020, 116, 84–104. [Google Scholar] [CrossRef]
- Oelhafen, K.; Shayota, B.J.; Muhleman, M.; Klaassen, Z.; Shoja, M.M.; Tubbs, R.S.; Loukas, M. Peritoneal bands: A review of anatomical distribution and clinical implications. Am. Surg. 2012, 78, 377–384. [Google Scholar] [CrossRef] [PubMed]
- de Paula, F.; Teshima, T.H.N.; Hsieh, R.; Souza, M.M.; Nico, M.M.S.; Lourenco, S.V. Overview of Human Salivary Glands: Highlights of Morphology and Developing Processes. Anat. Rec. 2017, 300, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Castelli, J.; Simon, A.; Louvel, G.; Henry, O.; Chajon, E.; Nassef, M.; Haigron, P.; Cazoulat, G.; Ospina, J.D.; Jegoux, F.; et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat. Oncol. 2015, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, T.; Arabi, A.; Feizi, S. Pterygium: An update on pathophysiology, clinical features, and management. Ther. Adv. Ophthalmol. 2021, 13, 25158414211020152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Tsai, C.F.; Tsai, M.C.; Chen, W.K.; Hsu, Y.W.; Lu, F.J. Anti-fibrotic effect of rosmarinic acid on inhibition of pterygium epithelial cells. Int. J. Ophthalmol. 2018, 11, 189–195. [Google Scholar] [PubMed]
- Cin, B.; Ciloglu, N.S.; Omar, S.; Kaya Terzi, N. Effect of Rosmarinic Acid and Alcohol on Fat Graft Survival in Rat Model. Aesthetic Plast. Surg. 2020, 44, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.W.; Wu, W.J.; Lu, C.W.; Wang, S.E.; Chuang, W.C.; Lee, M.C.; Wu, C.H. Traditional Chinese Medicine Yang-Gan-Wan Alleviated Experimental Hepatic Damage by Inhibiting Oxidation, Inflammation, and Apoptosis in Cell and Mouse Models. Evid. Based Complement. Altern. Med. 2021, 2021, 556352. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, Y.; Ito, M. Rosmarinic acid in Perilla frutescens and perilla herb analyzed by HPLC. J. Nat. Med. 2020, 74, 341–352. [Google Scholar] [CrossRef]
- Yong, C.; Zhang, Z.; Huang, G.; Yang, Y.; Zhu, Y.; Qian, L.; Tian, F.; Liu, L.; Wu, Q.; Xu, Z.; et al. Exploring the Critical Components and Therapeutic Mechanisms of Perilla frutescens L. in the Treatment of Chronic Kidney Disease via Network Pharmacology. Front. Pharmacol. 2021, 12, 717744. [Google Scholar] [CrossRef]
- Levsh, O.; Pluskal, T.; Carballo, V.; Mitchell, A.J.; Weng, J.K. Independent evolution of rosmarinic acid biosynthesis in two sister families under the Lamiids clade of flowering plants. J. Biol. Chem. 2019, 294, 15193–15205. [Google Scholar] [CrossRef]
- Lamichane, S.; Dahal Lamichane, B.; Kwon, S.M. Pivotal Roles of Peroxisome Proliferator-Activated Receptors (PPARs) and Their Signal Cascade for Cellular and Whole-Body Energy Homeostasis. Int. J. Mol. Sci. 2018, 19, 949. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Luo, L.; Yu, W.; Li, P.; Ou, D.; Liu, J.; Ma, H.; Sun, Q.; Liang, A.; Huang, C.; et al. PPARgamma phase separates with RXRalpha at PPREs to regulate target gene expression. Cell Discov. 2022, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Mihaylov, S.R.; Castelli, L.M.; Lin, Y.H.; Gul, A.; Soni, N.; Hastings, C.; Flynn, H.R.; Paun, O.; Dickman, M.J.; Snijders, A.P.; et al. The master energy homeostasis regulator PGC-1alpha exhibits an mRNA nuclear export function. Nat. Commun. 2023, 14, 5496. [Google Scholar] [CrossRef] [PubMed]
- Kokeny, G.; Calvier, L.; Hansmann, G. PPARgamma and TGFbeta-Major Regulators of Metabolism, Inflammation, and Fibrosis in the Lungs and Kidneys. Int. J. Mol. Sci. 2021, 22, 10431. [Google Scholar] [CrossRef] [PubMed]
- Gowans, G.J.; Hardie, D.G. AMPK: A cellular energy sensor primarily regulated by AMP. Biochem. Soc. Trans. 2014, 42, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Hardie, D.G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 2022, 24, 255–272. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Boo, Y.C. Natural Nrf2 Modulators for Skin Protection. Antioxidants 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Kuga, A.; Tsuchida, K.; Panda, H.; Horiuchi, M.; Otsuki, A.; Taguchi, K.; Katsuoka, F.; Suzuki, M.; Yamamoto, M. The beta-TrCP-Mediated Pathway Cooperates with the Keap1-Mediated Pathway in Nrf2 Degradation In Vivo. Mol. Cell Biol. 2022, 42, e0056321. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.Y.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.B.; Zhang, Z.Q.; Zhang, H.Y.; Hu, H.B. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful Plant Antioxidants: A New Biosustainable Approach to the Production of Rosmarinic Acid. Antioxidants 2020, 9, 1273. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 2018, 102, 7775–7793. [Google Scholar] [CrossRef]
- García-Melero, J.; López-Mitjavila, J.J.; García-Celma, M.J.; Rodriguez-Abreu, C.; Grijalvo, S. Rosmarinic Acid-Loaded Polymeric Nanoparticles Prepared by Low-Energy Nano-Emulsion Templating: Formulation, Biophysical Characterization, and In Vitro Studies. Materials 2022, 15, 4572. [Google Scholar] [CrossRef]
- Chung, C.H.; Jung, W.; Keum, H.; Kim, T.W.; Jon, S. Nanoparticles Derived from the Natural Antioxidant Rosmarinic Acid Ameliorate Acute Inflammatory Bowel Disease. Acs Nano 2020, 14, 6887–6896. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavaric, N.; Bozin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Min, J.B.; Chen, H.; Gong, Z.P.; Liu, X.; Wu, T.; Li, W.R.; Fang, J.S.; Huang, T.L.; Zhang, Y.F.; Zhao, W.; et al. Pharmacokinetic and Pharmacodynamic Properties of Rosmarinic Acid in Rat Cholestatic Liver Injury. Molecules 2018, 23, 2287. [Google Scholar] [CrossRef]




| Disease Model | Animals | Inducer | Test Materials and Doses | Literature |
|---|---|---|---|---|
| Liver fibrosis | Sprague Dawley rats | CCl4 | RA, 2.5, 5, or 10 mg kg−1, i.g. | [64] |
| Cholestatic liver fibrosis | C57BL/6 mice | Bile duct ligation | RA, 4 mg kg−1, i.p | [65] |
| Cholestatic liver injury | Sprague Dawley rats | Bile duct ligation | Glechoma hederacea extract, 500 or 2000 mg kg−1, p.o. | [66] |
| RA, 5 or 20 mg kg−1, p.o. | [67] | |||
| Liver fibrosis | Sprague Dawley rats | Thioacetamide | RA, 10 mg kg−1, p.o.; silymarin, 50 mg kg−1, p.o. | [68] |
| Nonalcoholic steatohepatitis | db/db mice | Methionine- and choline-deficient diet | Lemon balm (Melissa officinalis) extract, 200 mg kg−1, p.o.; RA, 10 or 30 mg kg−1, p.o. | [69] |
| Liver cirrhosis | C57BL/6 mice | CCl4 or high-fat choline-deficient L-amino acid-defined diet | RA, 30 mg kg−1, i.g. | [70] |
| Kidney ischemia/reperfusion injury | BALB/c mice | Bilateral renal ischemia | Wen-pi-tang-Hab-Wu-ling-san, 0.5, 2, 10, 50, or 100 mg/kg−1, p.o., pre- and post-ischemic treatments. | [71] |
| Wen-pi-tang-Hab-Wu-ling-san, 10 or 100 mg kg−1, p.o. | [72] | |||
| Kidney fibrosis | C57BL/6 mice | Unilateral ureteral obstruction | Wen-pi-tang-Hab-Wu-ling-san, 2, 10, or 50 mg kg−1, p.o. | [73] |
| Kidney fibrosis | Sprague Dawley rats | Unilateral ureteral obstruction | Elsholtzia ciliata extract, 300 or 500 mg kg−1, p.o.; captopril, 200 mg kg−1, p.o. | [74] |
| Kidney fibrosis | C57BL/6 mice | Unilateral ureteral obstruction | RA, 10 or 20 mg kg−1, o.g. | [75] |
| Nephrotoxicity | Swiss mice | CdCl2 | RA, 50 mg kg−1, p.o. | [76] |
| Diabetic nephropathy | Sprague Dawley rats | Streptozotocin | Lycopus lucidus extract, 3, 6, or 12 g kg−1 | [77] |
| Kidney fibrosis in heart failure | C57BL/6 mice | Transverse aortic constriction | Guanxinning injection, 3, 6, or 12 mL kg−1, i.v.; telmisartan, 6.1 mg/kg, o.g. | [78] |
| Myocardial infarction | Wistar rats | Isoproterenol | Basil (Ocimum basilicum) extract, 10, 20, or 40 mg kg−1, p.o. | [79] |
| Myocardial infarction | Sprague Dawley rats | Left anterior descending coronary artery ligation | Xia-Ku-Cao (Prunella vulgaris) extract, 200 or 400 mg kg−1, p.o. | [80] |
| Myocardial infarction | Sprague Dawley rats | Left anterior descending coronary artery ligation | RA, 50, 100, or 200 mg kg−1, p.o. | [81] |
| Myocardial infarction | Sprague Dawley rats | Left anterior descending coronary artery ligation | Hydrogel encapsulating RA nanoparticles, 100 μL per animal heart, s.c. | [82] |
| Long-term pressure overload | C57BL/6 mice | Aortic banding | RA, 100 mg kg−1 day−1, i.g. | [83] |
| Cardiotoxicity | Wistar rats | Doxorubicin | RA, 10, 20, or 40 mg kg−1, i.p.; vitamin E, 200 mg kg−1, i.p. | [84] |
| Lung fibrosis | Wistar rats | Bleomycin | Rosemary (Rosmarinus officinalis) leaf extract, 75 mg kg−1, i.p. | [85] |
| Lung fibrosis | Wistar rats | Bleomycin | RA, 5 mg kg−1, i.p.; carnosic acid, 5 mg kg−1, i.p.; vitamin E, 300 mg kg−1, i.p. | [86] |
| Lung fibrosis | Sprague Dawley rats | X-ray irradiation | RA, 30, 60, or 120 mg kg−1, i.g. | [87] |
| Asthma | BALB/c mice | Ovalbumin | Salvia miltiorrhiza water extract, 31.5 or 156 mg kg−1, p.o.; Salvia miltiorrhiza ethanol extract, 49.2 or 246 mg kg−1, p.o. | [88] |
| Asthma | Wistar rats | Ovalbumin | RA, 0.125, 0.250, or 0.500 mg mL−1 in drinking water; dexamethasone, 1.25 g mL−1 in drinking water. | [89] |
| Peritoneal adhesion | Wistar rats | Abdominal surgery | RA, 50 or 70 mg kg−1, 3 mL poured over the lesion site. | [90] |
| Post-surgical adhesion | Sprague Dawley rats | Midline laparotomy incision | PGAcys hydrogel; PGAcys/RA hydrogel; hyaluronate/carboxymethylcellulose. | [91] |
| Parotid gland injury | Sprague Dawley rats | X-ray irradiation | RA, 30, 60, or 120 mg kg−1, i.g.; amifostine, 250 mg kg−1, i.p. | [92] |
| Skin wound | Wistar rats | Full-thickness skin wound | 10% RA cream; 5% dexpanthenol cream, applied on the wound. | [93] |
| Skin wound | Sprague Dawley rats | Full-thickness skin wound | RA-grafted hydrogel, 50 μL, placed on the wound. | [94] |
| Herbal Prescription or Plant Name | Plants of Lamiaceae | Plants of Other Families | Literature |
|---|---|---|---|
| Yang-Gan-Wan | Angelica sinensis (Oliv.) Diels (Apiaceae); Plantago asiatica L. (Plantago depressa Willd.) (Plantaginaceae); Paeonia lactiflora Pall. (Paeoniaceae); Saposhnikovia divaricate (Turcz.) Schischk. (Apiaceae); Prinsepia utilis Royle (Rosaceae); Rehmanniae preparata (Orobanchaceae); Ligusticum scoticum L. (Apiaceae); Citrus aurantium L. (Rutaceae) | [65,118] | |
| Fuzheng Huayu recipe | Salvia miltiorrhiza Bunge | Cordyceps militaris (L.) Fr. (Cordycipitaceae); Prunus persica (L.) Batsch (Rosaceae); Gynostemma pentaphyllum (Thunb.) Makino (Cucurbitaceae); Pinus massoniana Lamb. (Pinaceae); Schisandrae Chinensis (Turcz.) Baill. (Magnoliaceae) | [101,102] |
| Wen-pi-tang-Hab-Wu-ling-san | Salvia miltiorrhiza Bunge; Perilla frutescens (L.) Britton | Codonopsis pilosula Franch. (Campanulaceae); Pinellia ternata (Thunb.) Makino (Araceae); Coptis chinensis Franch. (Ranunculaceae); Epimedium koreanum Nakai (Berberidaceae); Rheum palmatum L. (Polygonaceae); Glycyrrhiza uralensis Fisch. ex DC. (Fabaceae); Artemisia capillaris Thunb. (Asteraceae); Alisma plantago-aquatica var. orientale Samuels (Alismataceae); Wolfiporia extensa (Peck) Wolfiporia extensa (Peck) Ginns (Poria cocos (Schw.) Wolf (Polyporaceae); Atractylodes macrocephala Koidz. (Asteraceae); Polyporus umbellatus (Pers.) Fr. (Polyporaceae); Cinnamomum cassia (L.) J.Presl (Lauraceae) | [71,72,73] |
| Guanxinning injection | Salvia miltiorrhiza Bunge | Ligusticum chuanxiong Hort. (Apiaceae) | [78] |
| Ground ivy (gill-over-the-ground) | Glechoma hederacea L. | [66] | |
| Lemon balm | Melissa officinalis L. | [69] | |
| Xiang-Ru (Vietnamese balm) | Elsholtzia ciliata (Thunb.) Hyl. | [74] | |
| Ze-Lan (hirsute shiny bugleweed) | Lycopus lucidus Turcz. ex Benth. | [77] | |
| Basil | Ocimum basilicum L. | [79] | |
| Xia-Ku-Cao (self-heal) | Prunella vulgaris L. | [80] | |
| Rosemary | Salvia rosmarinus Spenn (Rosmarinus officinalis L.) | [85] | |
| Danshen (redroot sage) | Salvia miltiorrhiza Bunge | [88,101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boo, Y.C. Therapeutic Potential and Mechanisms of Rosmarinic Acid and the Extracts of Lamiaceae Plants for the Treatment of Fibrosis of Various Organs. Antioxidants 2024, 13, 146. https://doi.org/10.3390/antiox13020146
Boo YC. Therapeutic Potential and Mechanisms of Rosmarinic Acid and the Extracts of Lamiaceae Plants for the Treatment of Fibrosis of Various Organs. Antioxidants. 2024; 13(2):146. https://doi.org/10.3390/antiox13020146
Chicago/Turabian StyleBoo, Yong Chool. 2024. "Therapeutic Potential and Mechanisms of Rosmarinic Acid and the Extracts of Lamiaceae Plants for the Treatment of Fibrosis of Various Organs" Antioxidants 13, no. 2: 146. https://doi.org/10.3390/antiox13020146