Cocoa Shell Extract Reduces Blood Pressure in Aged Hypertensive Rats via the Cardiovascular Upregulation of Endothelial Nitric Oxide Synthase and Nuclear Factor (Erythroid-Derived 2)-like 2 Protein Expression
Abstract
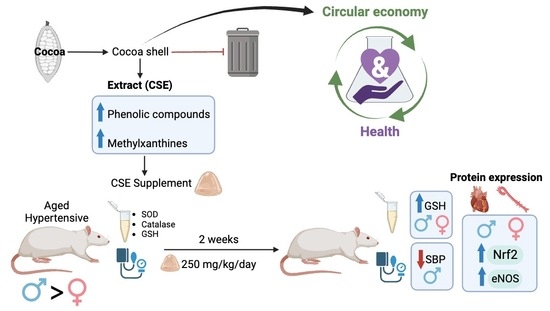
:1. Introduction
2. Materials and Methods
2.1. Experimental Model of Hypertension Induced by Fetal Undernutrition
2.2. Experimental Design
2.3. Blood Pressure Measurements
2.4. Supplementation Protocol
2.5. Plasma Antioxidants and Biomarkers of Oxidative Damage
2.6. Western Blotting
2.7. Study of Aorta Structure using Confocal Microscopy
2.8. Statistical Analysis
3. Results
3.1. Effect of CSE Supplementation on Systolic Blood Pressure in Aged MUN Rats
3.2. Effect of CSE Supplementation on Plasma Oxidative Status in Aged MUN Rats
3.3. Effect of CSE Supplementation on Nrf2 Protein Expression in Hearts and Aortas from Aged MUN Rats
3.4. Effect of CSE Supplementation on e-NOS Protein Expression in Hearts and Aortas from Aged MUN Rats
3.5. Effect of CSE Supplementation on Protein Expression of Antioxidant Enzymes in Heart and Aorta from Aged MUN Rats
3.6. Effect of CSE Supplementation on Aorta Structure from Aged MUN rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- Franco, C.; Sciatti, E.; Favero, G.; Bonomini, F.; Vizzardi, E.; Rezzani, R. Essential Hypertension and Oxidative Stress: Novel Future Perspectives. Int. J. Mol. Sci. 2022, 23, 14489. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Creus-Cuadros, A.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Estruch, R.; Gómez-Gracia, E.; Lapetra, J.; et al. Associations between Both Lignan and Yogurt Consumption and Cardiovascular Risk Parameters in an Elderly Population: Observations from a Cross-Sectional Approach in the PREDIMED Study. J. Acad. Nutr. Diet. 2017, 117, 609–622.e1. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Sievenpiper, J.L. Plant Polyphenols Lignans and Cardiovascular Disease. J. Am. Coll. Cardiol. 2021, 78, 679–682. [Google Scholar] [CrossRef]
- Aune, D. Plant Foods, Antioxidant Biomarkers, and the Risk of Cardiovascular Disease, Cancer, and Mortality: A Review of the Evidence. Adv. Nutr. 2019, 10, S404–S421. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose-Response Meta-Analysis. Mol. Nutr. Food Res. 2021, 65, e2001019. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, Supply Chain and Processing of Cocoa—A Review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Kiehbadroudinezhad, M. Environmental Impacts of Chocolate Production and Consumption. In Trends in Sustainable Chocolate Production; Galanakis, C.M., Ed.; Springer Cham: Chania, Greece, 2022; pp. 229–258. [Google Scholar]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa Shell and Its Compounds: Applications in the Food Industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Segovia, Á.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Extraction of Phenolic Compounds from Cocoa Shell: Modeling Using Response Surface Methodology and Artificial Neural Networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Mejia, E.G. Cocoa Shell Aqueous Phenolic Extract Preserves Mitochondrial Function and Insulin Sensitivity by Attenuating Inflammation between Macrophages and Adipocytes In Vitro. Mol. Nutr. Food Res. 2019, 63, 1801413. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Aguilera, Y.; Martin-Cabrejas, M.A.; Gonzalez de Mejia, E. Phytochemicals from the Cocoa Shell Modulate Mitochondrial Function, Lipid and Glucose Metabolism in Hepatocytes via Activation of FGF21/ERK, AKT, and MTOR Pathways. Antioxidants 2022, 11, 136. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; Ragusky, K.; Phuthong, S.; Ruvira, S.; Ramiro-Cortijo, D.; Cañas, S.; Rebollo-Hernanz, M.; Morales, M.D.; López de Pablo, Á.L.; Martín-Cabrejas, M.A.; et al. Vasoactive Properties of a Cocoa Shell Extract: Mechanism of Action and Effect on Endothelial Dysfunction in Aged Rats. Antioxidants 2022, 11, 429. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Salek, M.; Clark, C.C.T. Cocoa Consumption and Blood Pressure in Middle-Aged and Elderly Subjects: A Meta-Analysis. Curr. Hypertens. Rep. 2020, 22, 1. [Google Scholar] [CrossRef]
- Sun, Y.; Zimmermann, D.; De Castro, C.A.; Actis-Goretta, L. Dose-Response Relationship between Cocoa Flavanols and Human Endothelial Function: A Systematic Review and Meta-Analysis of Randomized Trials. Food Funct. 2019, 10, 6322–6330. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; López de Pablo, A.L.; García-Prieto, C.F.; Somoza, B.; Quintana-Villamandos, B.; Gómez de Diego, J.J.; Gutierrez-Arzapalo, P.Y.; Ramiro-Cortijo, D.; González, M.C.; Arribas, S.M. Long Term Effects of Fetal Undernutrition on Rat Heart. Role of Hypertension and Oxidative Stress. PLoS ONE 2017, 12, e0171544. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Arzapalo, P.Y.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Gil-Ortega, M.; Somoza, B.; de Pablo, Á.L.L.; González, M.D.C.; Arribas, S.M. Fetal Undernutrition Induces Resistance Artery Remodeling and Stiffness in Male and Female Rats Independent of Hypertension. Biomedicines 2020, 8, 424. [Google Scholar] [CrossRef]
- Ruvira, S.; Rodríguez-Rodríguez, P.; Cañas, S.; Ramiro-Cortijo, D.; Aguilera, Y.; Muñoz-Valverde, D.; Arribas, S.M. Evaluation of Parameters Which Influence Voluntary Ingestion of Supplements in Rats. Animals 2023, 13, 1827. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; Poasakate, A.; Ruvira-Hernando, S.; Gutierrez-Arzapalo, P.Y.; Böger, R.; Hannemann, J.; Lüneburg, N.; Arribas, S.M. Vascular Nitrosative Stress in Hypertension Induced by Fetal Undernutrition in Rats. J. Physiol. Biochem. 2023, 79, 555–568. [Google Scholar] [CrossRef]
- Phuthong, S.; Reyes-Hernández, C.G.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Gil-Ortega, M.; González-Blázquez, R.; González, M.C.; López de Pablo, A.L.; Arribas, S.M. Sex Differences in Placental Protein Expression and Efficiency in a Rat Model of Fetal Programming Induced by Maternal Undernutrition. Int. J. Mol. Sci. 2020, 22, 237. [Google Scholar] [CrossRef]
- Soares, T.F.; Oliveira, M.B.P.P. Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects. Molecules 2022, 27, 1625. [Google Scholar] [CrossRef]
- Cañas, S.; Rebollo-Hernanz, M.; Bermúdez-Gómez, P.; Rodríguez-Rodríguez, P.; Braojos, C.; Gil-Ramírez, A.; Benítez, V.; Aguilera, Y.; Martín-Cabrejas, M.A. Radical Scavenging and Cellular Antioxidant Activity of the Cocoa Shell Phenolic Compounds after Simulated Digestion. Antioxidants 2023, 12, 1007. [Google Scholar] [CrossRef]
- Liberale, L.; Badimon, L.; Montecucco, F.; Lüscher, T.F.; Libby, P.; Camici, G.G. Inflammation, Aging, and Cardiovascular Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wei, J.; Yi, Y.; Gong, Q.; Gao, J. Activation of Nrf2 Signaling: A Key Molecular Mechanism of Protection against Cardiovascular Diseases by Natural Products. Front. Pharmacol. 2022, 13, 1057918. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Krzemińska, J.; Wronka, M.; Młynarska, E.; Franczyk, B.; Rysz, J. Arterial Hypertension-Oxidative Stress and Inflammation. Antioxidants 2022, 11, 172. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, Age-Related Diseases and Oxidative Stress: What to Do Next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Moreno-Fernandez, J.; Ochoa, J.J.; Lopez-Frias, M.; Diaz-Castro, J. Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review. Nutrients 2020, 12, 3900. [Google Scholar] [CrossRef]
- Youssef, L.; Castellani, R.; Valenzuela-Alcaraz, B.; Sepulveda-Martinez, Á.; Crovetto, F.; Crispi, F. Cardiac Remodeling from the Fetus to Adulthood. J. Clin. Ultrasound 2023, 51, 249–264. [Google Scholar] [CrossRef]
- Gutiérrez-Arzapalo, P.Y.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; López de Pablo, Á.L.; López-Giménez, M.R.; Condezo-Hoyos, L.; Greenwald, S.E.; González, M.D.C.; Arribas, S.M. Role of Fetal Nutrient Restriction and Postnatal Catch-up Growth on Structural and Mechanical Alterations of Rat Aorta. J. Physiol. 2018, 596, 5791–5806. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; de Pablo, A.L.L.; Condezo-Hoyos, L.; Martín-Cabrejas, M.A.; Aguilera, Y.; Ruiz-Hurtado, G.; Gutierrez-Arzapalo, P.Y.; Ramiro-Cortijo, D.; Fernández-Alfonso, M.S.; González, M.D.C.; et al. Fetal Undernutrition Is Associated with Perinatal Sex-Dependent Alterations in Oxidative Status. J. Nutr. Biochem. 2015, 26, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.P.; Santana, A.; Baruqui, D.L.; Suraci, N. The Cardiovascular Effects of Chocolate. Rev. Cardiovasc. Med. 2018, 19, 123–127. [Google Scholar] [CrossRef]
- Dugo, L.; Tripodo, G.; Santi, L.; Fanali, C. Cocoa Polyphenols: Chemistry, Bioavailability and Effects on Cardiovascular Performance. Curr. Med. Chem. 2018, 25, 4903–4917. [Google Scholar] [CrossRef]
- Septianti, E.; Salengke; Langkong, J. Profile of Bioactive Compounds, Antioxidant and Aromatic Component from Several Clones of Cocoa Beans during Fermentation. IOP Conf. Ser. Earth Environ. Sci. 2020, 575, 012009. [Google Scholar] [CrossRef]
- Rodríguez-Artalejo, F.; López-García, E. Coffee Consumption and Cardiovascular Disease: A Condensed Review of Epidemiological Evidence and Mechanisms. J. Agric. Food Chem. 2018, 66, 5257–5263. [Google Scholar] [CrossRef] [PubMed]
- Surma, S.; Oparil, S. Coffee and Arterial Hypertension. Curr. Hypertens. Rep. 2021, 23, 38. [Google Scholar] [CrossRef]
- Borghi, C. Coffee and Blood Pressure: Exciting News! Blood Press. 2022, 31, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galán, A.; Prieto, A. Caffeine’s Vascular Mechanisms of Action. Int. J. Vasc. Med. 2010, 2010, 834060. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Role of Dietary Polyphenols in the Activity and Expression of Nitric Oxide Synthases: A Review. Antioxidants 2023, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Hida, M.; Hasegawa, M.; Matsumoto, T.; Kobayashi, T. Dietary Polyphenol Morin Rescues Endothelial Dysfunction in a Diabetic Mouse Model by Activating the Akt/ENOS Pathway. Mol. Nutr. Food Res. 2016, 60, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Masodsai, K.; Lin, Y.-Y.; Chaunchaiyakul, R.; Su, C.-T.; Lee, S.-D.; Yang, A.-L. Twelve-Week Protocatechuic Acid Administration Improves Insulin-Induced and Insulin-Like Growth Factor-1-Induced Vasorelaxation and Antioxidant Activities in Aging Spontaneously Hypertensive Rats. Nutrients 2019, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Juurlink, B.H.J.; Azouz, H.J.; Aldalati, A.M.Z.; AlTinawi, B.M.H.; Ganguly, P. Hydroxybenzoic Acid Isomers and the Cardiovascular System. Nutr. J. 2014, 13, 63. [Google Scholar] [CrossRef]
- Li, L.; Ma, H.; Zhang, Y.; Jiang, H.; Xia, B.; Sberi, H.A.; Elhefny, M.A.; Lokman, M.S.; Kassab, R.B. Protocatechuic Acid Reverses Myocardial Infarction Mediated by β-Adrenergic Agonist via Regulation of Nrf2/HO-1 Pathway, Inflammatory, Apoptotic, and Fibrotic Events. J. Biochem. Mol. Toxicol. 2023, 37, e23270. [Google Scholar] [CrossRef]
- Sundaresan, S.; John, S.; Paneerselvam, G.; Andiapppan, R.; Christopher, G.; Selvam, G.S. Gallic Acid Attenuates Cadmium Mediated Cardiac Hypertrophic Remodelling through Upregulation of Nrf2 and PECAM-1signalling in Rats. Environ. Toxicol. Pharmacol. 2021, 87, 103701. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guzmán, M.; Jiménez, R.; Sánchez, M.; Zarzuelo, M.J.; Galindo, P.; Quintela, A.M.; López-Sepúlveda, R.; Romero, M.; Tamargo, J.; Vargas, F.; et al. Epicatechin Lowers Blood Pressure, Restores Endothelial Function, and Decreases Oxidative Stress and Endothelin-1 and NADPH Oxidase Activity in DOCA-Salt Hypertension. Free Radic. Biol. Med. 2012, 52, 70–79. [Google Scholar] [CrossRef]
- Ramprasath, T.; Vasudevan, V.; Sasikumar, S.; Puhari, S.S.M.; Saso, L.; Selvam, G.S. Regression of Oxidative Stress by Targeting ENOS and Nrf2/ARE Signaling: A Guided Drug Target for Cardiovascular Diseases. Curr. Top. Med. Chem. 2015, 15, 857–871. [Google Scholar] [CrossRef]
- Mann, G.E.; Rowlands, D.J.; Li, F.Y.L.; de Winter, P.; Siow, R.C.M. Activation of Endothelial Nitric Oxide Synthase by Dietary Isoflavones: Role of NO in Nrf2-Mediated Antioxidant Gene Expression. Cardiovasc. Res. 2007, 75, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, V.; Scudiero, R.; Sica, R.; Lionetti, L.; Putti, R. Oxidative Stress and Mitochondrial Uncoupling Protein 2 Expression in Hepatic Steatosis Induced by Exposure to Xenobiotic DDE and High Fat Diet in Male Wistar Rats. PLoS ONE 2019, 14, e0215955. [Google Scholar] [CrossRef]
- Hass, D.T.; Barnstable, C.J. Uncoupling Proteins in the Mitochondrial Defense against Oxidative Stress. Prog. Retin. Eye Res. 2021, 83, 100941. [Google Scholar] [CrossRef]
- Yamashita, Y.; Okabe, M.; Natsume, M.; Ashida, H. Prevention Mechanisms of Glucose Intolerance and Obesity by Cacao Liquor Procyanidin Extract in High-Fat Diet-Fed C57BL/6 Mice. Arch. Biochem. Biophys. 2012, 527, 95–104. [Google Scholar] [CrossRef]
- Matsumura, Y.; Nakagawa, Y.; Mikome, K.; Yamamoto, H.; Osakabe, N. Enhancement of Energy Expenditure Following a Single Oral Dose of Flavan-3-Ols Associated with an Increase in Catecholamine Secretion. PLoS ONE 2014, 9, e112180. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and Vascular Function. Int. J. Mol. Sci. 2019, 20, 2155. [Google Scholar] [CrossRef]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, I.; Sankar, S.; Govindaraj, S. Ameliorative Effect of Epigallocatechin Gallate on Cardiac Hypertrophy and Fibrosis in Aged Rats. J. Cardiovasc. Pharmacol. 2018, 71, 65–75. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Suschek, C.V.; Wetzel, W.; Kröncke, K.-D.; Kolb-Bachofen, V. Nitric Oxide-Mediated Protection of Endothelial Cells from Hydrogen Peroxide Is Mediated by Intracellular Zinc and Glutathione. Am. J. Physiol. Cell Physiol. 2009, 296, C811-20. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of Glutathione Synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Tan, M.; Yin, Y.; Ma, X.; Zhang, J.; Pan, W.; Tan, M.; Zhao, Y.; Yang, T.; Jiang, T.; Li, H. Glutathione System Enhancement for Cardiac Protection: Pharmacological Options against Oxidative Stress and Ferroptosis. Cell Death Dis. 2023, 14, 131. [Google Scholar] [CrossRef]
- Kumar, P.; Liu, C.; Suliburk, J.; Hsu, J.W.; Muthupillai, R.; Jahoor, F.; Minard, C.G.; Taffet, G.E.; Sekhar, R. V Supplementing Glycine and N-Acetylcysteine (GlyNAC) in Older Adults Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Physical Function, and Aging Hallmarks: A Randomized Clinical Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 75–89. [Google Scholar] [CrossRef]
- Siepi, D.; Marchesi, S.; Lupattelli, G.; Paltriccia, R.; Vaudo, G.; Pirro, M.; Roscini, A.R.; Scarponi, A.M.; Sinzinger, H.; Mannarino, E. Postprandial Endothelial Impairment and Reduced Glutathione Levels in Postmenopausal Women. Ann. Nutr. Metab. 2002, 46, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, K.A.; Sekhar, R.V.; Granillo, A.; Reddy, A.; Medrano, G.; Heredia, C.P.; Entman, M.L.; Hamilton, D.J.; Li, S.; Reineke, E.; et al. Improved Cardiovascular Function in Old Mice After N-Acetyl Cysteine and Glycine Supplemented Diet: Inflammation and Mitochondrial Factors. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Osahon, O.W.; Sekhar, R. V GlyNAC (Glycine and N-Acetylcysteine) Supplementation in Mice Increases Length of Life by Correcting Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Abnormalities in Mitophagy and Nutrient Sensing, and Genomic Damage. Nutrients 2022, 14, 1114. [Google Scholar] [CrossRef] [PubMed]







| Antibody | Dilution | Company (City, Country) | MW (kDa) | Loading | %SDS-PAGE |
|---|---|---|---|---|---|
| e-NOS | 1:250 | BD Transduction (CA, USA) | 140 | 30 μg | 7% |
| p-eNOS (Ser 1177) | 1:250 | Cell Signaling Technology (MA, USA) | 140 | 30 μg | 7% |
| Nrf2 | 1:1000 | Abcam (Cambridge, UK) | 90 | 30 μg | 12% |
| p-Nrf2 (Ser 40) | 1:500 | Thermo Fisher Scientific (MA, USA) | 90 | 30 μg | 12% |
| SOD-2 | 1:1000 | Santa Cruz Biotech. (TX, USA) | 25 | 30 μg | 15% |
| Catalase | 1:1000 | Sigma-Aldrich (MO, USA) | 60 | 30 μg | 15% |
| HO-1 | 1:2000 | Sigma Aldrich (MO, USA) | 32 | 30 μg | 12% |
| UCP-2 | 1:250 | Santa Cruz Biotech. (TX, USA) | 33 | 30 μg | 12% |
| GADPH | 1:5000 | Cell Signalling Technology (MA, USA) | 37 | 30 μg | Various |
| Males (n = 5) | Females (n = 5) | p-Value | ||
|---|---|---|---|---|
| Basal (mmHg) | Post-CSE (mmHg) | Basal (mmHg) | Post-CSE (mmHg) | Group = 0.022 |
| 184.0 [180.0; 186.0] | 159.0 [157.0; 162.0] | 135.0 [132.0; 140.0] | 140.0 [134.0; 149.0] | Sex = 0.010 |
| Interaction = 0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruvira, S.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Martín-Trueba, M.; Martín-Cabrejas, M.A.; Arribas, S.M. Cocoa Shell Extract Reduces Blood Pressure in Aged Hypertensive Rats via the Cardiovascular Upregulation of Endothelial Nitric Oxide Synthase and Nuclear Factor (Erythroid-Derived 2)-like 2 Protein Expression. Antioxidants 2023, 12, 1698. https://doi.org/10.3390/antiox12091698
Ruvira S, Rodríguez-Rodríguez P, Ramiro-Cortijo D, Martín-Trueba M, Martín-Cabrejas MA, Arribas SM. Cocoa Shell Extract Reduces Blood Pressure in Aged Hypertensive Rats via the Cardiovascular Upregulation of Endothelial Nitric Oxide Synthase and Nuclear Factor (Erythroid-Derived 2)-like 2 Protein Expression. Antioxidants. 2023; 12(9):1698. https://doi.org/10.3390/antiox12091698
Chicago/Turabian StyleRuvira, Santiago, Pilar Rodríguez-Rodríguez, David Ramiro-Cortijo, María Martín-Trueba, María A. Martín-Cabrejas, and Silvia M. Arribas. 2023. "Cocoa Shell Extract Reduces Blood Pressure in Aged Hypertensive Rats via the Cardiovascular Upregulation of Endothelial Nitric Oxide Synthase and Nuclear Factor (Erythroid-Derived 2)-like 2 Protein Expression" Antioxidants 12, no. 9: 1698. https://doi.org/10.3390/antiox12091698