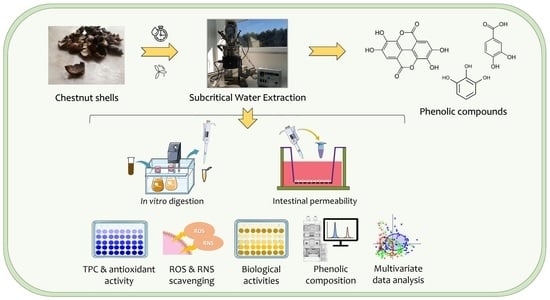
Simulated Gastrointestinal Digestion of Chestnut (Castanea sativa Mill.) Shell Extract Prepared by Subcritical Water Extraction: Bioaccessibility, Bioactivity, and Intestinal Permeability by In Vitro Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample
2.3. Preparation of C. sativa Shells Extract by Subcritical Water Extraction
2.4. In Vitro Simulated Gastrointestinal Digestion
2.5. Total Phenolic and Flavonoid Contents
2.6. In Vitro Antioxidant/Antiradical Activities
2.7. Reactive Oxygen and Nitrogen Species Counteracting Potential
2.8. Antioxidant Enzymes Activities and Lipid Peroxidation
2.9. In Vitro Biological Activities
2.9.1. Acetylcholinesterase Activity Inhibition
2.9.2. Amylase Activity Inhibition
2.10. Phenolic Profile by LC/DAD-ESI-MS
2.11. In Vitro Intestinal Permeability
2.12. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic and Flavonoids Contents
3.2. Bioaccessibility
3.3. Effects of In Vitro Digestion on Antioxidant/Antiradical Activity
3.4. In Vitro Radicals Scavenging Efficiency
3.5. Modulation of Antioxidant Enzymes’ Activities and Lipid Peroxidation
3.6. Inhibition of Acetylcholinesterase and α-Amylase Activities
3.7. Phenolic Composition by LC-DAD-ESI/MS
3.8. In Vitro Intestinal Permeability
3.9. Screening of Potential Differences by Multivariate Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto, D.; Cádiz-Gurrea, M.d.l.L.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Castanea sativa shells: A review on phytochemical composition, bioactivity and waste management approaches for industrial valorization. Food Res. Int. 2021, 144, 110364. [Google Scholar] [CrossRef]
- Pinto, D.; Cádiz-Gurrea, M.d.l.L.; Garcia, J.; Saavedra, M.J.; Freitas, V.; Costa, P.; Sarmento, B.; Delerue-Matos, C.; Rodrigues, F. From soil to cosmetic industry: Validation of a new cosmetic ingredient extracted from chestnut shells. Sustain. Mater. Technol. 2021, 29, e00309. [Google Scholar] [CrossRef]
- Pinto, D.; Cádiz-Gurrea, M.d.l.L.; Sut, S.; Ferreira, A.S.; Leyva-Jimenez, F.J.; Dall’Acqua, S.; Segura-Carretero, A.; Delerue-Matos, C.; Rodrigues, F. Valorisation of underexploited Castanea sativa shells bioactive compounds recovered by supercritical fluid extraction with CO2: A response surface methodology approach. J. CO2 Util. 2020, 40, 101194. [Google Scholar] [CrossRef]
- Pinto, D.; Silva, A.M.; Freitas, V.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Microwave-assisted extraction as a green technology approach to recover polyphenols from Castanea sativa shells. ACS Food Sci. Technol. 2021, 1, 229–241. [Google Scholar] [CrossRef]
- Pinto, D.; Almeida, A.; López-Yerena, A.; Pinto, S.; Sarmento, B.; Lamuela-Raventós, R.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Appraisal of a new potential antioxidants-rich nutraceutical ingredient from chestnut shells through in-vivo assays—A targeted metabolomic approach in phenolic compounds. Food Chem. 2023, 404, 134546. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; López-Yerena, A.; Almeida, A.; Sarmento, B.; Lamuela-Raventós, R.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Metabolomic insights into phenolics-rich chestnut shells extract as a nutraceutical ingredient—A comprehensive evaluation of its impacts on oxidative stress biomarkers by an in-vivo study. Food Res. Int. 2023, 170, 112963. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Moreira, M.M.; Švarc-Gajić, J.; Vallverdú-Queralt, A.; Brezo-Borjan, T.; Delerue-Matos, C.; Rodrigues, F. In-vitro gastrointestinal digestion of functional cookies enriched with chestnut shells extract: Effects on phenolic composition, bioaccessibility, bioactivity, and α-amylase inhibition. Food Biosci. 2023, 53, 102766. [Google Scholar] [CrossRef]
- Pinto, D.; Moreira, M.M.; Vieira, E.F.; Švarc-Gajić, J.; Vallverdú-Queralt, A.; Brezo-Borjan, T.; Delerue-Matos, C.; Rodrigues, F. Development and characterization of functional cookies enriched with chestnut shells extract as source of bioactive phenolic compounds. Foods 2023, 12, 640. [Google Scholar] [CrossRef]
- Martinelli, E.; Granato, D.; Azevedo, L.; Gonçalves, J.E.; Lorenzo, J.M.; Munekata, P.E.S.; Simal-Gandara, J.; Barba, F.J.; Carrillo, C.; Rajoka, M.S.R.; et al. Current perspectives in cell-based approaches towards the definition of the antioxidant activity in food. Trends Food Sci. Technol. 2021, 116, 232–243. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Álvarez-Parrilla, E.; de la Rosa, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Pinto, D.; Reis, J.; Silva, A.M.; Salazar, M.; Dall’Acqua, S.; Delerue-Matos, C.; Rodrigues, F. Valorisation of Salicornia ramosissima biowaste by a green approach—An optimizing study using response surface methodology. Sustain. Chem. Pharm. 2021, 24, 100548. [Google Scholar] [CrossRef]
- Silva, A.M.; Almeida, A.; Dall’Acqua, S.; Loschi, F.; Sarmento, B.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Insights into the 3D in vitro permeability and in vivo antioxidant protective effects of kiwiberry leaf extract: A step forward to human nutraceutical use. Int. J. Mol. Sci. 2022, 23, 14130. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Wang, J.; Chai, Z.; Zhang, X.; Wang, J.; Wang, N.; Huang, W. Impact of in vitro gastrointestinal digestion on rabbiteye blueberry anthocyanins and their absorption efficiency in Caco-2 cells. Food Biosci. 2023, 52, 102424. [Google Scholar] [CrossRef]
- Kashyap, P.; Riar, C.S.; Jindal, N. Effect of extraction methods and simulated in vitro gastrointestinal digestion on phenolic compound profile, bio-accessibility, and antioxidant activity of Meghalayan cherry (Prunus nepalensis) pomace extracts. LWT—Food Sci. Technol. 2022, 153, 112570. [Google Scholar] [CrossRef]
- Tu, F.; Xie, C.; Li, H.; Lei, S.; Li, J.; Huang, X.; Yang, F. Effect of in vitro digestion on chestnut outer-skin and inner-skin bioaccessibility: The relationship between biotransformation and antioxidant activity of polyphenols by metabolomics. Food Chem. 2021, 363, 130277. [Google Scholar] [CrossRef] [PubMed]
- Durán-Castañeda, A.C.; Cardenas-Castro, A.P.; Pérez-Jiménez, J.; Pérez-Carvajal, A.M.; Sánchez-Burgos, J.A.; Mateos, R.; Sáyago-Ayerdi, S.G. Bioaccessibility of phenolic compounds in Psidium guajava L. varieties and P. friedrichsthalianum Nied. after gastrointestinal digestion. Food Chem. 2023, 400, 134046. [Google Scholar] [CrossRef]
- Ed Nignpense, B.; Latif, S.; Francis, N.; Blanchard, C.; Santhakumar, A.B. The impact of simulated gastrointestinal digestion on the bioaccessibility and antioxidant activity of purple rice phenolic compounds. Food Biosci. 2022, 47, 101706. [Google Scholar] [CrossRef]
- Muñoz-Fariña, O.; López-Casanova, V.; García-Figueroa, O.; Roman-Benn, A.; Ah-Hen, K.; Bastias-Montes, J.M.; Quevedo-León, R.; Ravanal-Espinosa, M.C. Bioaccessibility of phenolic compounds in fresh and dehydrated blueberries (Vaccinium corymbosum L.). Food Chem. 2023, 2, 100171. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Campos-Vega, R.; Gaytán-Martínez, M.; Preciado-Ortiz, R.; Mendoza, S.; Loarca-Piña, G. Bioaccessibility and antioxidant activity of free phenolic compounds and oligosaccharides from corn (Zea mays L.) and common bean (Phaseolus vulgaris L.) chips during in vitro gastrointestinal digestion and simulated colonic fermentation. Food Res. Int. 2017, 100, 304–311. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, Q.; Zhao, H.; Li, X.; Sang, S.; McClements, D.J.; Long, J.; Jin, Z.; Wang, J.; Qiu, C. Bioaccessibility and bioavailability of phytochemicals: Influencing factors, improvements, and evaluations. Food Hydrocoll. 2023, 135, 108165. [Google Scholar] [CrossRef]
- Schmidt, L.; Prestes, O.D.; Augusti, P.R.; Moreira, J.C.F. Phenolic compounds and contaminants in olive oil and pomace—A narrative review of their biological and toxic effects. Food Biosci. 2023, 53, 102626. [Google Scholar] [CrossRef]
- Galli, F. Interactions of polyphenolic compounds with drug disposition and metabolism. Curr. Drug Metab. 2007, 8, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Mercatante, D.; Ansorena, D.; Taticchi, A.; Astiasarán, I.; Servili, M.; Rodriguez-Estrada, M.T. Effects of in vitro digestion on the antioxidant activity of three phenolic extracts from olive mill wastewaters. Antioxidants 2023, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Peanparkdee, M.; Borompichaichartkul, C.; Iwamoto, S. Bioaccessibility and antioxidant activity of phenolic acids, flavonoids, and anthocyanins of encapsulated Thai rice bran extracts during in vitro gastrointestinal digestion. Food Chem. 2021, 361, 130161. [Google Scholar] [CrossRef]
- Radünz, M.; Camargo, T.M.; dos Santos Hackbart, H.C.; Blank, J.P.; Hoffmann, J.F.; Stefanello, F.M.; Zavareze, E.d.R. Encapsulation of broccoli extract by electrospraying: Influence of in vitro simulated digestion on phenolic and glucosinolate contents, and on antioxidant and antihyperglycemic activities. Food Chem. 2021, 339, 128075. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Lu, P.; Barrow, C.; Dunshea, F.R.; Suleria, H.A.R. Bioaccessibility and bioactivities of phenolic compounds from roasted coffee beans during in vitro digestion and colonic fermentation. Food Chem. 2022, 386, 132794. [Google Scholar] [CrossRef]
- Ketsawatsakul, U.; Whiteman, M.; Halliwell, B. A reevaluation of the peroxynitrite scavenging activity of some dietary phenolics. Biochem. Biophys. Res. Commun. 2000, 279, 692–699. [Google Scholar] [CrossRef]
- Cynthia, I.F.; Hery, S.; Akhmad, D. Antibacterial and antioxidant activities of pyrogallol and synthetic pyrogallol dimer. Res. J. Chem. Environ. 2018, 22, 39–47. [Google Scholar]
- Kumar, A.; Kaushik, P.; Incerpi, S.; Pedersen, J.Z.; Goel, S.; Prasad, A.K.; Rohil, V.; Parmar, V.S.; Saso, L.; Len, C. Evaluation of the free radical scavenging activities of ellagic acid and ellagic acid peracetate by EPR spectrometry. Molecules 2021, 26, 4800. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Santangelo, C.; D’Archivio, M.; Li Volti, G.; Giovannini, C.; Galvano, F. Protocatechuic acid and human disease prevention: Biological activities and molecular mechanisms. Curr. Med. Chem. 2012, 19, 2901–2917. [Google Scholar] [CrossRef] [PubMed]
- Lameirão, F.; Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Sut, S.; Dall’Acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Green-sustainable recovery of phenolic and antioxidant compounds from industrial chestnut shells using ultrasound-assisted extraction: Optimization and evaluation of biological activities in vitro. Antioxidants 2020, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Aboul-Enein, H.Y.; Kruk, I.; Kładna, A.; Lichszteld, K.; Michalskaet, T. Scavenging effects of phenolic compounds on reactive oxygen species. Biopolymers 2007, 86, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.; Oppolzer, D.; Gouvinhas, I.; Silva, A.M.; Barros, A.I.R.N.A.; Domínguez-Perles, R. New grape stems’ isolated phenolic compounds modulate reactive oxygen species, glutathione, and lipid peroxidation in vitro: Combined formulations with vitamins C and E. Fitoterapia 2017, 120, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef]
- López-Yerena, A.; Domínguez-López, I.; Vallverdú-Queralt, A.; Pérez, M.; Jáuregui, O.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Metabolomics technologies for the identification and quantification of dietary phenolic compound metabolites: An overview. Antioxidants 2021, 10, 846. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U. Modification of enzymatic and non-enzymatic in vitro oxidative defence system by bioaccessible phytonutrients of selected spices. LWT—Food Sci. Technol. 2014, 57, 434–441. [Google Scholar] [CrossRef]
- Vahid, F.; Wagener, L.; Leners, B.; Bohn, T. Pro- and antioxidant effect of food items and matrices during simulated in vitro digestion. Foods 2023, 12, 1719. [Google Scholar] [CrossRef]
- Paulo Farias, D.; Araújo, F.F.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; Catharino, R.R.; Pastore, G.M. Effect of in vitro digestion on the bioaccessibility and bioactivity of phenolic compounds in fractions of Eugenia pyriformis fruit. Food Res. Int. 2021, 150, 110767. [Google Scholar] [CrossRef]
- Murugan, R.; Chandran, R.; Parimelazhagan, T. Effect of in vitro simulated gastrointestinal digestion of Phoenix loureirii on polyphenolics, antioxidant and acetylcholinesterase inhibitory activities. LWT—Food Sci. Technol. 2016, 74, 363–370. [Google Scholar] [CrossRef]
- Jagadeesan, G.; Muniyandi, K.; Manoharan, A.L.; Nataraj, G.; Thangaraj, P. Understanding the bioaccessibility, α-amylase and α-glucosidase enzyme inhibition kinetics of Allmania nodiflora (L.) R.Br. ex Wight polyphenols during in vitro simulated digestion. Food Chem. 2022, 372, 131294. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Ozturk Sarikaya, S.B. Acethylcholinesterase inhibitory potential and antioxidant properties of pyrogallol. J. Enzyme Inhib. Med. Chem. 2015, 30, 761–766. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.; Yang, L.; Xue, Q.; Yu, M.; Yao, F.; Sun, L.; Liuet, Y. Identification and inhibitory activities of ellagic acid- and kaempferol-derivatives from Mongolian oak cups against α-glucosidase, α-amylase and protein glycation linked to type II diabetes and its complications and their influence on HepG2 cells’ viability. Arab. J. Chem. 2018, 11, 1247–1259. [Google Scholar]
- Ferreira, A.S.; Silva, A.M.; Pinto, D.; Moreira, M.M.; Ferraz, R.; Švarc-Gajić, J.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. New perspectives on the sustainable employment of chestnut shells as active ingredient against oral mucositis: A first screening. Int. J. Mol. Sci. 2022, 23, 14956. [Google Scholar] [CrossRef]
- de Vasconcelos, M.d.C.B.M.; Bennett, R.N.; Quideau, S.; Jacquet, R.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Evaluating the potential of chestnut (Castanea sativa Mill.) fruit pericarp and integument as a source of tocopherols, pigments and polyphenols. Ind. Crops Prod. 2010, 31, 301–311. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Tomás-Barberán, F.A.; Iglesias-Aguirre, C.E.; Giménez-Bastida, J.A.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagitannins, urolithins, and neuroprotection: Human evidence and the possible link to the gut microbiota. Mol. Aspects Med. 2023, 89, 101109. [Google Scholar] [CrossRef]
- Kang, I.; Buckner, T.; Shay, N.F.; Gu, L.; Chung, S. Improvements in metabolic health with consumption of ellagic acid and subsequent conversion into urolithins: Evidence and mechanisms. Adv. Nutr. 2016, 7, 961–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimundo, A.F.; Ferreira, S.; Tomás-Barberán, F.A.; Santos, C.N.; Menezes, R. Urolithins: Diet-derived bioavailable metabolites to tackle diabetes. Nutrients 2021, 13, 4285. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Z.; Wang, X.; Yan, X.; Guo, Q.; Yue, Y.; Yue, T.; Yuan, Y. The bioaccessibility, bioavailability, bioactivity, and prebiotic effects of phenolic compounds from raw and solid-fermented mulberry leaves during in vitro digestion and colonic fermentation. Food Res. Int. 2023, 165, 112493. [Google Scholar] [CrossRef]
- Guan, H.; Li, P.; Wang, Q.; Zeng, F.; Wang, D.; Zhou, M.; Zhou, M.; He, X.; Liao, S.; Pan, W. Systematically exploring the chemical ingredients and absorbed constituents of Polygonum capitatum in hyperuricemia rat plasma using UHPLC-Q-Orbitrap HRMS. Molecules 2022, 27, 3521. [Google Scholar] [CrossRef] [PubMed]
- Daré, R.G.; Oliveira, M.M.; Truiti, M.C.T.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Abilities of protocatechuic acid and its alkyl esters, ethyl and heptyl protocatechuates, to counteract UVB-induced oxidative injuries and photoaging in fibroblasts L929 cell line. J. Photochem. Photobiol. B 2020, 203, 111771. [Google Scholar] [CrossRef]
- Araújo, F.; Sarmento, B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int. J. Pharm. 2013, 458, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Addepalli, R.; Netzel, M.; Tinggi, U.; Fletcher, M.; Sultanbawa, Y.; Osborne, S. In vitro bioaccessibility and intestinal absorption of selected bioactive compounds in Terminalia ferdinandiana. Front. Nutr. 2022, 8, 818195. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.E.; Cremonini, E.; Fraga, C.G.; Oteiza, P.I. Ellagic acid protects Caco-2 cell monolayers against inflammation-induced permeabilization. Free Radic. Biol. Med. 2020, 152, 776–786. [Google Scholar] [CrossRef]
- Mao, X.; Wu, L.-F.; Zhao, H.-J.; Liang, W.-Y.; Chen, W.-J.; Han, S.-X.; Qi, Q.; Cui, Y.-P.; Li, S.; Yang, G.-H.; et al. Transport of corilagin, gallic acid, and ellagic acid from Fructus phyllanthi tannin fraction in Caco-2 cell monolayers. Evid. Based Complement. Altern. Med. 2016, 2016, 9205379. [Google Scholar] [CrossRef] [Green Version]
- Whitley, A.C.; Stoner, G.D.; Darby, M.V.; Walle, T. Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid—Extensive binding to protein and DNA. Biochem. Pharmacol. 2003, 66, 907–915. [Google Scholar] [CrossRef]
- Cilla, A.; Rodrigo, M.J.; Zacarías, L.; De Ancos, B.; Sánchez-Moreno, C.; Barberá, R.; Alegría, A. Protective effect of bioaccessible fractions of citrus fruit pulps against H2O2-induced oxidative stress in Caco-2 cells. Food Res. Int. 2018, 103, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The two-way polyphenols-microbiota interactions and their effects on obesity and related metabolic diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| In Vitro Simulated Digestion | Undigested CS Extract | |||
|---|---|---|---|---|
| Oral Digest | Gastric Digest | Intestinal Digest | ||
| TPC (mg GAE/g DW) | 87.86 ± 3.06 c | 127.44 ± 5.34 b | 141.77 ± 12.81 b | 362.28 ± 21.10 a |
| Phenolics recovery (%) | 24.28 ± 1.02 b | 35.21 ± 1.29 a | 39.33 ± 5.16 a | − |
| TFC (mg CE/g DW) | 31.42 ± 1.02 c | 29.87 ± 1.06 c | 39.16 ± 2.08 b | 103.87 ± 3.74 a |
| Flavonoids recovery (%) | 30.26 ± 1.02 b | 28.78 ± 1.41 b | 37.72 ± 2.05 a | − |
| ABTS (mg AAE/g DW) | 104.36 ± 2.40 c | 366.45 ± 11.84 b | 427.64 ± 6.27 b | 728.52 ± 45.35 a |
| DPPH (mg TE/g DW) | 187.56 ± 4.16 c | 276.15 ± 28.29 b | 328.73 ± 10.10 b | 784.58 ± 29.02 a |
| FRAP (µmol FSE/g DW) | 979.02 ± 104.46 c | 1537.68 ± 87.14 b | 1562.75 ± 88.74 b | 7659.96 ± 94.23 a |
| Reactive Oxygen Species | Reactive Nitrogen Species | |||||
|---|---|---|---|---|---|---|
| O2●− | H2O2 | HOCl | ROO● | ONOO− | ||
| IC50 (µg/mL) | µmol TE/mg DW | In Presence of NaHCO3 IC50 (µg/mL) | In Absence of NaHCO3 IC50 (µg/mL) | |||
| Oral digest | 9.88 ± 1.27 *,3 | 14.34 ± 2.28 *,1 | 22.70 ± 1.12 *,3 | 0.04 ± 0.01 b | 18.37 ± 0.42 *,3 | 16.40 ± 0.59 *,3 |
| Gastric digest | 17.85 ± 1.32 *,2 | 34.82 ± 3.11 *,2 | 60.17 ± 0.23 *,2 | 0.13 ± 0.00 b | 55.61 ± 1.21 *,2 | 50.81 ± 0.96 *,2 |
| Intestinal digest | 60.65 ± 2.07 *,1 | 56.59 ± 1.66 *,3 | 90.10 ± 0.85 *,1 | 0.21 ± 0.01 b | 83.72 ± 1.63 *,1 | 74.55 ± 0.54 *,1 |
| Undigested CS extract # | 12.92 ± 0.34 #,b | 114.51 ± 3.63 a | 0.79 ± 0.06 #,b | 0.32 ± 0.01 #,b | 1.75 ± 0.07 a | 1.88 ± 0.04 a |
| Positive controls | ||||||
| Catechin | 48.21 ± 4.79 a | 20.78 ± 0.75 c | 0.37 ± 0.01 c | 1.81 ± 0.12 a | 0.23 ± 0.01 b | 0.16 ± 0.02 b |
| Gallic acid | 10.95 ± 1.40 b | 106.03 ± 0.93 b | 1.81 ± 0.02 a | 1.08 ± 0.10 a | 0.29 ± 0.02 b | 0.15 ± 0.02 b |
| In Vitro Simulated Digestion | Undigested CS Extract | |||
|---|---|---|---|---|
| Oral Digest | Gastric Digest | Intestinal Digest | ||
| CAT (nmol/min/g DW) | 80.64 ± 10.34 c | 213.42 ± 29.67 b | 208.13 ± 23.38 b | 1706.14 ± 44.96 a |
| GSH-Px (µmol/min/g DW) | 160.77 ± 5.68 b | 352.89 ± 21.60 b | 633.44 ± 13.64 b | 9083.60 ± 824.20 a |
| SOD (µmol/min/g DW) | 13.65 ± 1.89 c | 249.52 ± 10.71 b | 521.31 ± 42.83 a | n.d. |
| LPO (nmol MDA/mg DW) | 3.95 ± 0.27 a | 1.30 ± 0.09 b | 0.40 ± 0.02 c | 1.58 ± 0.06 b |
| N° | Rt (min) | Compounds | [M−H]− | Fragments | Amount (µg/g DW) | Amount (µg/mg DW) | ||
|---|---|---|---|---|---|---|---|---|
| Oral | Gastric | Intestinal | Undigested CS Extract | |||||
| 1 | 5.63 | Pyrogallol–protocatechuic acid derivative | 261.1 | 261 243 203 177 137 | 199.20 ± 16.97 b | 165.81 ± 22.35 b | 310.32 ± 28.68 a | n.d. |
| 2 | 16.8 | Ellagic acid | 301.0 | 301 284 257 229 185 157 129 | 440.80 ± 14.71 b | 741.50 ± 92.55 a | 206.40 ± 65.89 c | 10.50 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, D.; Silva, A.M.; Dall’Acqua, S.; Sut, S.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Simulated Gastrointestinal Digestion of Chestnut (Castanea sativa Mill.) Shell Extract Prepared by Subcritical Water Extraction: Bioaccessibility, Bioactivity, and Intestinal Permeability by In Vitro Assays. Antioxidants 2023, 12, 1414. https://doi.org/10.3390/antiox12071414
Pinto D, Silva AM, Dall’Acqua S, Sut S, Vallverdú-Queralt A, Delerue-Matos C, Rodrigues F. Simulated Gastrointestinal Digestion of Chestnut (Castanea sativa Mill.) Shell Extract Prepared by Subcritical Water Extraction: Bioaccessibility, Bioactivity, and Intestinal Permeability by In Vitro Assays. Antioxidants. 2023; 12(7):1414. https://doi.org/10.3390/antiox12071414
Chicago/Turabian StylePinto, Diana, Ana Margarida Silva, Stefano Dall’Acqua, Stefania Sut, Anna Vallverdú-Queralt, Cristina Delerue-Matos, and Francisca Rodrigues. 2023. "Simulated Gastrointestinal Digestion of Chestnut (Castanea sativa Mill.) Shell Extract Prepared by Subcritical Water Extraction: Bioaccessibility, Bioactivity, and Intestinal Permeability by In Vitro Assays" Antioxidants 12, no. 7: 1414. https://doi.org/10.3390/antiox12071414