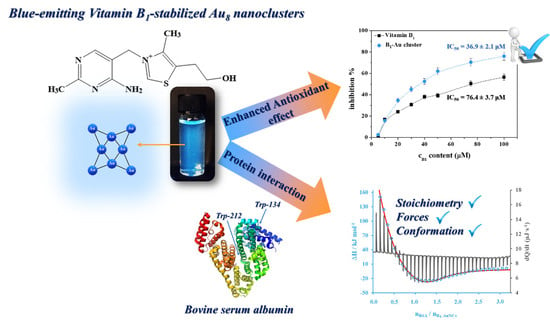
Promising Bioactivity of Vitamin B1-Au Nanocluster: Structure, Enhanced Antioxidant Behavior, and Serum Protein Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation Protocol of B1-Au NCs
2.3. Antioxidant Measurements
2.4. Experimental Conditions for the Investigation of Protein Interaction by Spectrofluorometry and Isotherm Titration Calorimetry
2.5. Instruments
3. Results and Discussion
3.1. Structural Characterization of the Fluorescent B1-Au NCs
3.2. Antioxidant Studies
3.3. Interaction of B1-Au NCs with Bovine Serum Albumin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ungor, D.; Csapó, E.; Kismárton, B.; Juhász, Á.; Dékány, I. Nucleotide-Directed Syntheses of Gold Nanohybrid Systems with Structure-Dependent Optical Features: Selective Fluorescence Sensing of Fe3+ Ions. Colloids Surf. B Biointerfaces 2017, 155, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungor, D.; Dékány, I.; Csapó, E. Reduction of Tetrachloroaurate(III) Ions With Bioligands: Role of the Thiol and Amine Functional Groups on the Structure and Optical Features of Gold Nanohybrid Systems. Nanomaterials 2019, 9, 1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, Y.; Fu, L.; Ye, L.; Li, B.; Xu, X.; Xing, X.; He, J.; Song, Y.; Leng, C.; Guo, Y.; et al. Protein-Directed Synthesis of Highly Monodispersed, Spherical Gold Nanoparticles and Their Applications in Multidimensional Sensing. Sci. Rep. 2016, 6, 28900. [Google Scholar] [CrossRef] [PubMed]
- Xavier, P.L.; Chaudhari, K.; Baksi, A.; Pradeep, T. Protein-Protected Luminescent Noble Metal Quantum Clusters: An Emerging Trend in Atomic Cluster Nanoscience. Nano Rev. 2012, 3, 14767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, P.; Nandi, N.; Barnwal, N.; Sahu, K. Mercaptopropionic Acid-Assisted Synthesis of Green and Blue Emissive Copper Nanoclusters for Multimodal Sensing, Logic Gate, and White Light Applications. Mater. Today Chem. 2023, 27, 101341. [Google Scholar] [CrossRef]
- Chen, T.-H.; Tseng, W.-L. (Lysozyme Type VI)-Stabilized Au8 Clusters: Synthesis Mechanism and Application for Sensing of Glutathione in a Single Drop of Blood. Small 2012, 8, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Su, Z.; Tu, Y.; Yan, J. Determination of Dopamine Based on Its Enhancement of Gold-Silver Nanocluster Fluorescence. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119519. [Google Scholar] [CrossRef] [PubMed]
- Ungor, D.; Szilágyi, I.; Csapó, E. Yellow-Emitting Au/Ag Bimetallic Nanoclusters with High Photostability for Detection of Folic Acid. J. Mol. Liq. 2021, 338, 116695. [Google Scholar] [CrossRef]
- Ungor, D.; Horváth, K.; Dékány, I.; Csapó, E. Red-Emitting Gold Nanoclusters for Rapid Fluorescence Sensing of Tryptophan Metabolites. Sens. Actuators B Chem. 2019, 288, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Miao, H.; Yang, X. Glutathione-Stabilized Cu Nanoclusters as Fluorescent Probes for Sensing pH and Vitamin B1. Talanta 2015, 144, 488–495. [Google Scholar] [CrossRef]
- Shang, L.; Stockmar, F.; Azadfar, N.; Nienhaus, G.U. Intracellular Thermometry by Using Fluorescent Gold Nanoclusters. Angew. Chem. Int. Ed. 2013, 52, 11154–11157. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Surface Ligand Chemistry of Gold Nanoclusters Determines Their Antimicrobial Ability. Chem. Mater. 2018, 30, 2800–2808. [Google Scholar] [CrossRef]
- Jindal, S.; Gopinath, P. Exploration of Connexin-43 Modulating, Multifunctional Silver Nanocluster-Hydrogel System for Theranostic Management of Cancer. Mater. Today Chem. 2022, 26, 101213. [Google Scholar] [CrossRef]
- Li, D.; Wang, Z.; Kumari, B.; Mei, X.; Wang, Z.Y. Anti-Amyloid Nanoclusters for the Treatment of Brain Hazards Associated with Incurable Neurodegenerative Diseases. Mater. Today Chem. 2023, 27, 101256. [Google Scholar] [CrossRef]
- Qi, F.; Huang, H.; Wang, M.; Rong, W.; Wang, J. Applications of Antioxidants in Dental Procedures. Antioxidants 2022, 11, 2492. [Google Scholar] [CrossRef]
- Alam, M.W.; Al Qahtani, H.S.; Souayeh, B.; Ahmed, W.; Albalawi, H.; Farhan, M.; Abuzir, A.; Naeem, S. Novel Copper-Zinc-Manganese Ternary Metal Oxide Nanocomposite as Heterogeneous Catalyst for Glucose Sensor and Antibacterial Activity. Antioxidants 2022, 11, 1064. [Google Scholar] [CrossRef]
- Cortesi, R.; Esposito, E.; Drechsler, M.; Pavoni, G.; Cacciatore, I.; Sguizzato, M.; Di Stefano, A. L-Dopa Co-Drugs in Nanostructured Lipid Carriers: A Comparative Study. Mater. Sci. Eng. C 2017, 72, 168–176. [Google Scholar] [CrossRef]
- Hallan, S.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Montesi, L.; Cortesi, R.; Björklund, S.; Ruzgas, T.; Esposito, E. The Potential of Caffeic Acid Lipid Nanoparticulate Systems for Skin Application: In Vitro Assays to Assess Delivery and Antioxidant Effect. Nanomaterials 2021, 11, 171. [Google Scholar] [CrossRef]
- Sguizzato, M.; Pepe, A.; Baldisserotto, A.; Barbari, R.; Montesi, L.; Drechsler, M.; Mariani, P.; Cortesi, R. Niosomes for Topical Application of Antioxidant Molecules: Design and In Vitro Behavior. Gels 2023, 9, 107. [Google Scholar] [CrossRef]
- Sicurella, M.; Sguizzato, M.; Cortesi, R.; Huang, N.; Simelière, F.; Montesi, L.; Marconi, P.; Esposito, E. Mangiferin-Loaded Smart Gels for HSV-1 Treatment. Pharmaceutics 2021, 13, 1323. [Google Scholar] [CrossRef]
- Katana, B.; Kókai, K.P.; Sáringer, S.; Szerlauth, A.; Takács, D.; Szilágyi, I. The Influence of Solvents and Colloidal Particles on the Efficiency of Molecular Antioxidants. Antioxidants 2022, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.M.; Barrias, C.C.; Gomes, P.; Martins, M.C.L. Smart Biomaterial-Based Systems for Intrinsic Stimuli-Responsive Chronic Wound Management. Mater. Today Chem. 2021, 22, 100623. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC Assays Comparison to Measure the Antioxidant Capacity of Food Products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Edwards, K.A.; Tu-Maung, N.; Cheng, K.; Wang, B.; Baeumner, A.J.; Kraft, C.E. Thiamine Assays—Advances, Challenges, and Caveats. ChemistryOpen 2017, 6, 178–191. [Google Scholar] [CrossRef]
- Wood, D.M.; Ashcroft, N.W. Quantum Size Effects in the Optical Properties of Small Metallic Particles. Phys. Rev. B 1982, 25, 6255–6274. [Google Scholar] [CrossRef]
- Rincon, L.; Hasmy, A.; Marquez, M.; Gonzalez, C. A Perturbatively Corrected Tight-Binding Method with Hybridization: Application to Gold Nanoparticles. Chem. Phys. Lett. 2011, 503, 171–175. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, C.; Yu, M.; Liu, J. Different Sized Luminescent Gold Nanoparticles. Nanoscale 2012, 4, 4073. [Google Scholar] [CrossRef]
- Yang, T.-Q.; Peng, B.; Shan, B.-Q.; Zong, Y.-X.; Jiang, J.-G.; Wu, P.; Zhang, K. Origin of the Photoluminescence of Metal Nanoclusters: From Metal-Centered Emission to Ligand-Centered Emission. Nanomaterials 2020, 10, 261. [Google Scholar] [CrossRef] [Green Version]
- Bubeshko, N.N.; Stsiapura, V.I.; Stepuro, I.I. Fluorescent Properties of Thiochrome in Solvents of Different Polarity. J. Appl. Spectrosc. 2011, 78, 337–343. [Google Scholar] [CrossRef]
- Yamabe, S.; Tsuchida, N.; Yamazaki, S. How Is Vitamin B1 Oxidized to Thiochrome? Elementary Processes Revealed by a DFT Study. Org. Biomol. Chem. 2021, 19, 4529–4536. [Google Scholar] [CrossRef]
- Wakchaure, P.D.; Ganguly, B. Unraveling the Role of π-Stacking Interactions in Ligand Binding to the Thiamine Pyrophosphate Riboswitch with High-Level Quantum Chemical Calculations and Docking Study. J. Phys. Chem. B 2022, 126, 1076–1084. [Google Scholar] [CrossRef]
- Battistoni, C.; Mattogno, G.; Cariati, F.; Naldini, L.; Sgamellotti, A. XPS Photoelectron Spectra of Cluster Compounds of Gold. Inorg. Chim. Acta 1977, 24, 207–210. [Google Scholar] [CrossRef]
- Shchukarev, A.; Korolkov, D. XPS Study of Group IA Carbonates. Open Chem. 2004, 2, 347–362. [Google Scholar] [CrossRef] [Green Version]
- Jansen, R.J.J.; van Bekkum, H. XPS of Nitrogen-Containing Functional Groups on Activated Carbon. Carbon 1995, 33, 1021–1027. [Google Scholar] [CrossRef]
- Butler, I.S.; Kawai, N.T.; Yining, H.; Louloudi, M.; Hadjiliadis, N. Effect of High Pressure on the Infrared Spectra of the Thiamine Enzyme ‘Active Aldehyde’ Intermediate 2-(α-Hydroxybenzyl)Thiamine Chloride (HBT) and the Mercury(II) Complex, Hg(HBT)Cl3. Inorg. Chim. Acta 1992, 196, 119–122. [Google Scholar] [CrossRef]
- Sethiya, A.; Soni, J.; Manhas, A.; Jha, P.C.; Agarwal, S. Green and Highly Efficient MCR Strategy for the Synthesis of Pyrimidine Analogs in Water via C–C and C–N Bond Formation and Docking Studies. Res. Chem. Intermed. 2021, 47, 4477–4496. [Google Scholar] [CrossRef]
- Adeyemo, A.; Kolawole, G.; Oderinde, R. A Cobalt(II) Complex of Vitamin B1. J. Coord. Chem. 1986, 15, 181–184. [Google Scholar] [CrossRef]
- Archibong, E.; Adeyemo, A.; Aoki, K.; Yamazaki, H. Thiamine-Metal Ion and Thiamine-Anion Interactions. Crystal Structure of Cu(Thiamine)Br2. Inorg. Chim. Acta 1989, 156, 77–83. [Google Scholar] [CrossRef]
- Hu, N.-H.; Aoki, K.; Adeyemo, A.O.; Williams, G.N. Metal Ion and Anion Coordination in the Thiamine–[PtII(NO2)4]2− System. Structures of a Metal Complex, Pt(Thiamine)(NO2)3, and Two Salts, (H-Thiamine)[Pt(NO2)4]·2H2O and (Thiamine Monophosphate)2[Pt(NO2)4]·2H2O. Inorg. Chim. Acta 2001, 325, 9–19. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Ilanchelian, M. Simple and Visual Approach for Highly Selective Biosensing of Vitamin B1 Based on Glutathione Coated Silver Nanoparticles as a Colorimetric Probe. Sens. Actuators B Chem. 2017, 244, 380–386. [Google Scholar] [CrossRef]
- Lin, L.; Wang, J.; Liu, W.; Luo, Y.; Xiao, Y.; Wang, Y. Rapid and Visual Readout of Vitamin B1 Based on the Electrostatic Interaction Induced Aggregation of Gold Nanoparticles. RSC Adv. 2018, 8, 35850–35854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, G.D.; Metzler, D.E. Structures of Thiamine in Basic Solution 1. J. Am. Chem. Soc. 1957, 79, 4386–4391. [Google Scholar] [CrossRef]
- Bera, N.; Kiran Nandi, P.; Hazra, R.; Sarkar, N. Aggregation Induced Emission of Surface Ligand Controlled Gold Nanoclusters Employing Imidazolium Surface Active Ionic Liquid and PH Sensitivity. J. Photochem. Photobiol. A Chem. 2023, 437, 114471. [Google Scholar] [CrossRef]
- Lukienko, P.I.; Mel’nichenko, N.G.; Zverinskii, I.V.; Zabrodskaya, S.V. Antioxidant Properties of Thiamine. Bull. Exp. Biol. Med. 2000, 130, 874–876. [Google Scholar] [CrossRef]
- Gombár, G.; Ungor, D.; Samu, G.F.; Dömötör, O.; Csapó, E. Synthesis and Characterization of Novel Blue-Emitting Nicotinamide-Gold Nanoclusters with “Chain-Breaker” Antioxidant Property. J. Mol. Liq. 2022, 359, 119372. [Google Scholar] [CrossRef]
- Higashi-Okai, K.; Nagino, H.; Yamada, K.; Okai, Y. Antioxidant and Prooxidant Activities of B Group Vitamins in Lipid Peroxidation. J. UOEH 2006, 28, 359–368. [Google Scholar] [CrossRef]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidant Activity of Nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef]
- Strambini, G.B.; Gabellieri, E. Phosphorescence Properties and Protein Structure Surrounding Tryptophan Residues in Yeast, Pig, and Rabbit Glyceraldehyde-3-Phosphate Dehydrogenase. Biochemistry 1989, 28, 160–166. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2010; Volume 1961, ISBN 978-0-387-31278-1/978-0-387-46312-4. [Google Scholar]
- Scatchard, G. The Attractions of Proteins for Small Molecules and Ions. Ann. N. Y. Acad. Sci. 1949, 51, 660–672. [Google Scholar] [CrossRef]
- Sandu, N.; Chilom, C.G.; David, M.; Florescu, M. Evaluation of the Interaction of Levothyroxine with Bovine Serum Albumin Using Spectroscopic and Molecular Docking Studies. J. Biomol. Struct. Dyn. 2022, 40, 1139–1151. [Google Scholar] [CrossRef]
- Shang, L.; Brandholt, S.; Stockmar, F.; Trouillet, V.; Bruns, M.; Nienhaus, G.U. Effect of Protein Adsorption on the Fluorescence of Ultrasmall Gold Nanoclusters. Small 2012, 8, 661–665. [Google Scholar] [CrossRef]
- Shang, L.; Yang, L.; Seiter, J.; Heinle, M.; Brenner-Weiss, G.; Gerthsen, D.; Nienhaus, G.U. Nanoparticles Interacting with Proteins and Cells: A Systematic Study of Protein Surface Charge Effects. Adv. Mater. Interfaces 2014, 1, 1300079. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Khorsandi, K. Interaction of Vitamin B1 with Bovine Serum Albumin Investigation Using Vitamin B1-Selective Electrode: Potentiometric and Molecular Modeling Study. J. Biomol. Struct. Dyn. 2016, 34, 1903–1910. [Google Scholar] [CrossRef]
- Mallappa, M.; Shivakumar, A.; Gowda, B.G.; Nageshbabu, R. Binding Study of Thiamine Hydrochloride to Bovine Serum Albumin: Spectroscopic and Molecular Modeling Methods. J. Chem. Pharm. Res. 2017, 9, 85–91. [Google Scholar]
- Molodenskiy, D.; Shirshin, E.; Tikhonova, T.; Gruzinov, A.; Peters, G.; Spinozzi, F. Thermally Induced Conformational Changes and Protein–Protein Interactions of Bovine Serum Albumin in Aqueous Solution under Different PH and Ionic Strengths as Revealed by SAXS Measurements. Phys. Chem. Chem. Phys. 2017, 19, 17143–17155. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.-Y.; Song, L.-N.; Zhao, Y.; Zang, F.-L.; Zhao, Z.-H.; Chen, N.-H.; Xu, X.-J.; Wang, C.-J. Spectroscopic Study on the Interaction between Naphthalimide-Polyamine Conjugates and Bovine Serum Albumin (BSA). Molecules 2015, 20, 16491–16523. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of Protein Association Reactions: Forces Contributing to Stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Wiseman, T.; Williston, S.; Brandts, J.F.; Lin, L.-N. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal. Biochem. 1989, 179, 131–137. [Google Scholar] [CrossRef]





| First Binding Site | Second Binding Site | |
|---|---|---|
| Ka (M−1) | 6.1 × 105 ± 8.4 × 104 | 1.6 × 105 ± 2.4 × 104 |
| ΔG (kJ·mol−1) | −32.99 ± 0.34 | −29.73 ± 0.36 |
| ΔH (kJ·mol−1) | 779.90 ± 23.64 | −97.36 ± 4.14 |
| ΔS (J·mol−1·K−1) | 2728 ± 0.00 | −227 ± 0.00 |
| N | 0.20 ± 0.00 | 1.30 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungor, D.; Gombár, G.; Juhász, Á.; Samu, G.F.; Csapó, E. Promising Bioactivity of Vitamin B1-Au Nanocluster: Structure, Enhanced Antioxidant Behavior, and Serum Protein Interaction. Antioxidants 2023, 12, 874. https://doi.org/10.3390/antiox12040874
Ungor D, Gombár G, Juhász Á, Samu GF, Csapó E. Promising Bioactivity of Vitamin B1-Au Nanocluster: Structure, Enhanced Antioxidant Behavior, and Serum Protein Interaction. Antioxidants. 2023; 12(4):874. https://doi.org/10.3390/antiox12040874
Chicago/Turabian StyleUngor, Ditta, Gyöngyi Gombár, Ádám Juhász, Gergely F. Samu, and Edit Csapó. 2023. "Promising Bioactivity of Vitamin B1-Au Nanocluster: Structure, Enhanced Antioxidant Behavior, and Serum Protein Interaction" Antioxidants 12, no. 4: 874. https://doi.org/10.3390/antiox12040874