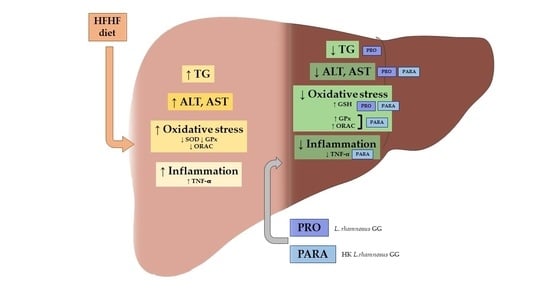
Beneficial Effects of Viable and Heat-Inactivated Lactobacillus rhamnosus GG Administration on Oxidative Stress and Inflammation in Diet-Induced NAFLD in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Liver Triacylglycerol Content and Serum Transaminases
2.3. Parameters Related to Oxidative Stress in Liver
2.3.1. Lipid Peroxidation Measurement
2.3.2. Total Antioxidant Capacity Determination
2.3.3. Determination of Nonenzymatic Antioxidant Glutathione
2.3.4. Superoxide Dismutase Activity (SOD; EC 1.15.1.1)
2.3.5. Catalase (CAT; EC 1.11.1.6)
2.3.6. Glutathione Peroxidase (GPx; EC 1.11.1.9)
2.3.7. Determination of Total Proteins
2.4. Parameters Related to Inflammation in Liver
IL-1β and TNF-α Determination
2.5. Parameters Related to DNA Damage and Cell Death by Immunoblotting
2.6. Statistical Analysis
3. Results
3.1. Body and Liver Weights, Hepatic Triglyceride Content and Serum Transaminase Levels
3.2. Parameters Related to Oxidative Stress in Liver
3.3. Parameters Related to Inflammation in Liver
3.4. Parameters Related to DNA Damage and Cell Death by Immunoblotting
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The Prevalence and Incidence of NAFLD Worldwide: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gordillo, K.; Shah, R.; Muriel, P. Oxidative Stress and Inflammation in Hepatic Diseases: Current and Future Therapy. Oxid. Med. Cell. Longev. 2017, 2017, 3140673. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Chen, Y.; Li, X.; Lu, Y. The Role and Mechanism of Oxidative Stress and Nuclear Receptors in the Development of NAFLD. Oxid. Med. Cell. Longev. 2021, 2021, 6889533. [Google Scholar] [CrossRef]
- Alsaif, F.; Al-hamoudi, W.; Alotaiby, M.; Alsadoon, A.; Almayouf, M.; Almadany, H.; Abuhaimed, J.; Ghufran, N.; Merajuddin, A.; Ali Khan, I. Molecular Screening via Sanger Sequencing of the Genetic Variants in Non-Alcoholic Fatty Liver Disease Subjects in the Saudi Population: A Hospital-Based Study. Metabolites 2022, 12, 1240. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a Continuum: From Obesity to Metabolic Syndrome and Diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inci, M.K.; Park, S.-H.; Helsley, R.N.; Attia, S.L.; Softic, S. Fructose Impairs Fat Oxidation: Implications for the Mechanism of Western Diet-Induced NAFLD. J. Nutr. Biochem. 2022, 114, 109224. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and Sugar: A Major Mediator of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, A.; Bonelli, P.; Tuccillo, F.M.; Goldfine, I.D.; Evans, J.L.; Buonaguro, F.M.; Mancini, A. Role of Gut Microbiota and Oxidative Stress in the Progression of Non-Alcoholic Fatty Liver Disease to Hepatocarcinoma: Current and Innovative Therapeutic Approaches. Redox Biol. 2018, 15, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Cortez-Pinto, H. Cell Death and Nonalcoholic Steatohepatitis: Where Is Ballooning Relevant? Expert Rev. Gastroenterol. Hepatol. 2011, 5, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Briskey, D.; Heritage, M.; Jaskowski, L.A.; Peake, J.; Gobe, G.; Subramaniam, V.N.; Crawford, D.; Campbell, C.; Vitetta, L. Probiotics Modify Tight-Junction Proteins in an Animal Model of Nonalcoholic Fatty Liver Disease. Therap. Adv. Gastroenterol. 2016, 9, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arellano-García, L.; Portillo, M.P.; Martínez, J.A.; Milton-Laskibar, I. Usefulness of Probiotics in the Management of NAFLD: Evidence and Involved Mechanisms of Action from Preclinical and Human Models. Int. J. Mol. Sci. 2022, 23, 3167. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S129–S134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Microb. Cell Fact. 2020, 19. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Liu, J.R. Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients 2020, 12, 2557. [Google Scholar] [CrossRef]
- Owens, J.A.; Saeedi, B.J.; Naudin, C.R.; Hunter-Chang, S.; Barbian, M.E.; Eboka, R.U.; Askew, L.; Darby, T.M.; Robinson, B.S.; Jones, R.M. Lactobacillus rhamnosus GG Orchestrates an Antitumor Immune Response. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1311–1327. [Google Scholar] [CrossRef]
- Li, N.; Russell, W.M.; Douglas-Escobar, M.; Hauser, N.; Lopez, M.; Neu, J. Live and Heat-Killed Lactobacillus rhamnosus GG: Effects on Proinflammatory and Anti-Inflammatory Cytokines/Chemokines in Gastrostomy-Fed Infant Rats. Pediatr. Res. 2009, 66, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, N.; Caicedo, R.; Neu, J. Alive and Dead Lactobacillus rhamnosus GG Decrease Tumor Necrosis Factor-Alpha-Induced Interleukin-8 Production in Caco-2 Cells. J. Nutr. 2005, 135, 1752–1756. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Perumpail, B.J.; Cholankeril, R.; Yoo, E.R.; Kim, D.; Ahmed, A. An Overview of Dietary Interventions and Strategies to Optimize the Management of Non-Alcoholic Fatty Liver Disease. Diseases 2017, 5, 23. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Hu, J.; Nie, S.; Xiong, T.; Xie, M. Intervention of Five Strains of Lactobacillus on Obesity in Mice Induced by High-Fat Diet. J. Funct. Foods 2020, 72, 104078. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Kuppusamy, P.; Srisesharam, S.; Lee, J.C.; Sivanesan, R.; Kim, D.; Choi, K.C. Positive Metabolic Effects of Selected Probiotic Bacteria on Diet-Induced Obesity in Mice Are Associated with Improvement of Dysbiotic Gut Microbiota. FASEB J. 2020, 34, 12289–12307. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; de Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.J.; Jung, A.H.; Joo Suh, H.; Choi, H.S.; Kim, H. Lactiplantibacillus plantarum K8-Based Paraprobiotics Prevents Obesity and Obesity-Induced Inflammatory Responses in High Fat Diet-Fed Mice. Food Res. Int. 2022, 155, 111066. [Google Scholar] [CrossRef]
- Liu, Z.-S.; Li, P.-L.; Ku, Y.-W.; Chen, P.-W. Oral Administration of Recombinant Lactoferrin-Expressing Probiotics Ameliorates Diet-Induced Lipid Accumulation and Inflammation in Non-Alcoholic Fatty Liver Disease in Mice. Microorganisms 2022, 10, 2215. [Google Scholar] [CrossRef]
- Chen, X.X.; Xu, Y.Y.; Wu, R.; Chen, Z.; Fang, K.; Han, Y.X.; Yu, Y.; Huang, L.L.; Peng, L.; Ge, J.F. Resveratrol Reduces Glucolipid Metabolic Dysfunction and Learning and Memory Impairment in a NAFLD Rat Model: Involvement in Regulating the Imbalance of Nesfatin-1 Abundance and Copine 6 Expression. Front. Endocrinol. 2019, 10, 434. [Google Scholar] [CrossRef]
- Huang, Y.; Lang, H.; Chen, K.; Zhang, Y.; Gao, Y.; Ran, L.; Yi, L.; Mi, M.; Zhang, Q. Resveratrol Protects against Nonalcoholic Fatty Liver Disease by Improving Lipid Metabolism and Redox Homeostasis via the PPARα Pathway. Appl. Physiol. Nutr. Metab. 2020, 45, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, C.; Zhao, S.; Wang, X.; Wang, J.; Zhang, H.; Wang, Y.; Zhao, G. Probiotic Bifidobacterium lactis V9 Attenuates Hepatic Steatosis and Inflammation in Rats with Non-Alcoholic Fatty Liver Disease. AMB Express 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, L.; Liu, Q.; Li, F.; Zhang, L.; Zhu, F.; Shao, T.; Barve, S.; Chen, Y.; Li, X.; et al. Fibroblast Growth Factor 21 Is Required for the Therapeutic Effects of Lactobacillus rhamnosus GG against Fructose-Induced Fatty Liver in Mice. Mol. Metab. 2019, 29, 145–157. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic Steatohepatitis: Association of Insulin Resistance and Mitochondrial Abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef]
- Browning, J.D.; Horton, J.D. Molecular Mediators of Hepatic Steatosis and Liver Injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Auger, C.; Alhasawi, A.; Contavadoo, M.; Appanna, V.D. Dysfunctional Mitochondrial Bioenergetics and the Pathogenesis of Hepatic Disorders. Front. Cell Dev. Biol. 2015, 3, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, S.; Hamada, A.; Jobu, K.; Yokota, J.; Onogawa, M.; Kyotani, S.; Miyamura, M.; Saibara, T.; Onishi, S.; Nishioka, Y. Effects of Eriobotrya Japonica Seed Extract on Oxidative Stress in Rats with Non-Alcoholic Steatohepatitis. J. Pharm. Pharmacol. 2010, 62, 241–246. [Google Scholar] [CrossRef]
- Song, L.; Qu, D.; Zhang, Q.; Jiang, J.; Zhou, H.; Jiang, R.; Li, Y.; Zhang, Y.; Yan, H. Phytosterol Esters Attenuate Hepatic Steatosis in Rats with Non-Alcoholic Fatty Liver Disease Rats Fed a High-Fat Diet. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Dornas, W.C.; de Lima, W.G.; dos Santos, R.C.; da Costa Guerra, J.F.; de Souza, M.O.; Silva, M.; Souza e Silva, L.; Diniz, M.F.; Silva, M.E. High Dietary Salt Decreases Antioxidant Defenses in the Liver of Fructose-Fed Insulin-Resistant Rats. J. Nutr. Biochem. 2013, 24, 2016–2022. [Google Scholar] [CrossRef] [Green Version]
- Martinez, O.D.M.; Theodoro, J.M.V.; Grancieri, M.; Toledo, R.C.L.; de Barros, F.A.R.; Tako, E.; Queiroz, V.A.V.; Martino, H.S.D. Dry Heated Sorghum BRS 305 Hybrid Flour as a Source of Resistant Starch and Tannins Improves Inflammation and Oxidative Stress in Wistar Rats Fed with a High-Fat High-Fructose Diet. Food Funct. 2021, 12, 8738–8746. [Google Scholar] [CrossRef]
- Abdelmoneim, D.; El-Adl, M.; El-Sayed, G.; El-Sherbini, E.S. Protective Effect of Fenofibrate against High-Fat–High-Fructose Diet Induced Non-Obese NAFLD in Rats. Fundam. Clin. Pharmacol. 2021, 35, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, L.; Zhu, Y.; Hou, D.; Li, Y.; Guo, X.; Wang, Y.; Olatunji, O.J.; Wan, P.; Gong, K. Kukoamine B Ameliorate Insulin Resistance, Oxidative Stress, Inflammation and Other Metabolic Abnormalities in High-Fat/High-Fructose-Fed Rats. Diabetes Metab. Syndr. Obes. 2020, 13, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Feillet-Coudray, C.; Fouret, G.; Vigor, C.; Bonafos, B.; Jover, B.; Blachnio-Zabielska, A.; Rieusset, J.; Casas, F.; Gaillet, S.; Landrier, J.F.; et al. Long-Term Measures of Dyslipidemia, Inflammation, and Oxidative Stress in Rats Fed a High-Fat/High-Fructose Diet. Lipids 2019, 54, 81–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as Potential Antioxidants: A Systematic Review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grajek, W.; Olejnik, A.; Sip, A. Probiotics, Prebiotics and Antioxidants as Functional Foods. Acta Biochim. Pol. 2005, 52, 665–671. [Google Scholar] [CrossRef]
- Song, M.W.; Jang, H.J.; Kim, K.T.; Paik, H.D. Probiotic and Antioxidant Properties of Novel Lactobacillus. brevis. KCCM 12203P Isolated from Kimchi and Evaluation of Immune-Stimulating Activities of Its Heat-Killed Cells in RAW 264.7 Cells. J. Microbiol. Biotechnol. 2019, 29, 1894–1903. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, Y.S.; Kim, S.M.; Park, G.S.; Lee, Y.H.; Jeong, D.Y.; Kang, J.; Lee, H.J. Beneficial Effects of Lactobacillus plantarum Strains on Non-Alcoholic Fatty Liver Disease in High Fat/High Fructose Diet-Fed Rats. Nutrients 2020, 12, 542. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Woo, K.J.; Kim, M.A.; Hong, J.; Kim, J.; Kim, S.H.; Han, K.-l.; Iwasa, M.; Kim, T.J. Heat-Killed Enterococcus faecalis Prevents Adipogenesis and High Fat Diet-Induced Obesity by Inhibition of Lipid Accumulation through Inhibiting C/EBP-α; and PPAR-γ; in the Insulin Signaling Pathway. Nutrients 2022, 14, 1308. [Google Scholar] [CrossRef]
- Shaker, M.E. The Contribution of Sterile Inflammation to the Fatty Liver Disease and the Potential Therapies. Biomed. Pharmacother. 2022, 148, 112789. [Google Scholar] [CrossRef]
- Astarini, F.D.; Ratnasari, N.; Wasityastuti, W. Update on Non-Alcoholic Fatty Liver Disease-Associated Single Nucleotide Polymorphisms and Their Involvement in Liver Steatosis, Inflammation, and Fibrosis: A Narrative Review. Iran. Biomed. J. 2022, 26, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Arrese, M.; Cabrera, D.; Kalergis, A.M.; Feldstein, A.E. Innate Immunity and Inflammation in NAFLD/NASH. Dig. Dis. Sci. 2016, 61, 1294–1303. [Google Scholar] [CrossRef] [Green Version]
- Golabi, P.; Rhea, L.; Henry, L.; Younossi, Z.M. Hepatocellular Carcinoma and Non-Alcoholic Fatty Liver Disease. Hepatol. Int. 2019, 13, 688–694. [Google Scholar] [CrossRef]
- Massoud, O.; Charlton, M. Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Clin. Liver Dis. 2018, 22, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Daugherity, E.K.; Balmus, G.; al Saei, A.; Moore, E.S.; Abi Abdallah, D.; Rogers, A.B.; Weiss, R.S.; Maurer, K.J. The DNA Damage Checkpoint Protein ATM Promotes Hepatocellular Apoptosis and Fibrosis in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Cell Cycle 2012, 11, 1918–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Zhao, H.; Dong, L.; Zhen, Y.F.; Xing, H.Y.; Ma, H.J.; Song, G.Y. Resveratrol Ameliorates High-Fat Diet-Induced Insulin Resistance and Fatty Acid Oxidation via ATM-AMPK Axis in Skeletal Muscle. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9117–9125. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 Cleavage Fragments: Signatures of Cell-Death Proteases in Neurodegeneration. Cell. Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Matsuoka, S.; Yamazaki, M.; Shibata, T.; Nirei, K.; Takahashi, H.; Kaneko, T.; Fujisawa, M.; Higuchi, T.; Nakamura, H.; et al. Apoptosis and Non-Alcoholic Fatty Liver Diseases. World J. Gastroenterol. 2018, 24, 2661–2672. [Google Scholar] [CrossRef]
- Karahan, N.; Işler, M.; Koyu, A.; Karahan, A.G.; Başyiǧt Kiliç, G.; Çiriş, I.M.; Sütçü, R.; Onaran, I.; Çam, H.; Keskin, M. Effects of Probiotics on Methionine Choline Deficient Diet-Induced Steatohepatitis in Rats. Turk. J. Gastroenterol. 2012, 23, 110–121. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [Green Version]
- Ragland, S.A.; Criss, A.K. From Bacterial Killing to Immune Modulation: Recent Insights into the Functions of Lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| STD | HFHF | |
|---|---|---|
| Total energy (kcal/g) | 3.9 | 4.5 |
| Composition by energy% | ||
| Carbohydrates | 63.9 | 40 |
| Fructose | - | 10 |
| Proteins | 20.3 | 20 |
| Lipids | 15.8 | 40 |
| C | HFHF | PRO | PARA | ANOVA | |
|---|---|---|---|---|---|
| Final body weight (g) | 407 ± 14 b | 469 ± 14 a | 435 ± 14 ab | 436 ± 12 ab | p < 0.01 |
| Food intake (g) | 20.3 ± 0.5 | 21.4 ± 0.4 | 20.2 ± 0.6 | 20.3 ± 0.5 | NS |
| Liver weight (g) | 11.8 ± 1.0 b | 21.5 ± 1.2 a | 19.0 ± 0.9 a | 18.8 ± 0.7 a | p < 0.001 |
| Liver TG (mg/g tissue) | 42.7 ± 1.7 c | 130.5 ± 4.6 a | 108.2 ± 4.4 b | 119.4 ± 4.6 ab | p < 0.05 |
| ALT (U/L) | 13.6 ± 3.1 b | 46.6 ± 11.5 a | 32.3 ± 2.6 a | 27.9 ± 3.2 a | p < 0.05 |
| AST (U/L) | 43.9 ± 1.8 b | 101.3 ± 21.6 a | 56.5 ± 6.5 ab | 61.0 ± 5.0 a | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arellano-García, L.; Trepiana, J.; Martínez, J.A.; Portillo, M.P.; Milton-Laskibar, I. Beneficial Effects of Viable and Heat-Inactivated Lactobacillus rhamnosus GG Administration on Oxidative Stress and Inflammation in Diet-Induced NAFLD in Rats. Antioxidants 2023, 12, 717. https://doi.org/10.3390/antiox12030717
Arellano-García L, Trepiana J, Martínez JA, Portillo MP, Milton-Laskibar I. Beneficial Effects of Viable and Heat-Inactivated Lactobacillus rhamnosus GG Administration on Oxidative Stress and Inflammation in Diet-Induced NAFLD in Rats. Antioxidants. 2023; 12(3):717. https://doi.org/10.3390/antiox12030717
Chicago/Turabian StyleArellano-García, Laura, Jenifer Trepiana, J. Alfredo Martínez, María P. Portillo, and Iñaki Milton-Laskibar. 2023. "Beneficial Effects of Viable and Heat-Inactivated Lactobacillus rhamnosus GG Administration on Oxidative Stress and Inflammation in Diet-Induced NAFLD in Rats" Antioxidants 12, no. 3: 717. https://doi.org/10.3390/antiox12030717