Antioxidant Materials in Oral and Maxillofacial Tissue Regeneration: A Narrative Review of the Literature
Abstract
:1. Introduction
2. Antioxidant Materials
2.1. Natural Antioxidants
2.2. Synthetic Antioxidants
3. Mechanisms of Antioxidants’ Activity
3.1. Free Radical Scavenging
3.1.1. Scavenging Superoxide
3.1.2. Scavenging Hydroxyl Radical
3.1.3. More Stable Radical Scavenging
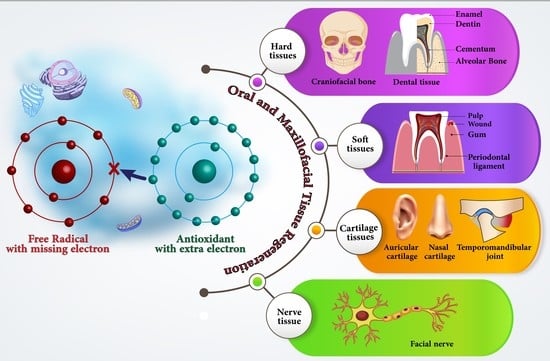
4. Role of Antioxidant Materials in the Tissue Regeneration Process
4.1. Hard Tissues in the Craniofacial and Alveolar Area
4.1.1. Craniofacial Bone Regeneration

4.1.2. Alveolar Bone Regeneration
4.2. Dental Hard Tissue Regeneration
4.2.1. Enamel Regeneration
4.2.2. Dentine Regeneration
4.2.3. Cementum Regeneration
4.3. Oral Soft Tissue Regeneration
4.3.1. Periodontal Soft Tissue Regeneration
4.3.2. Oral Wound Healing
4.4. Dental Pulp Tissue
4.5. Cartilage Tissue
4.5.1. TMJ Cartilage
4.5.2. Auricular and Nasal Cartilage Regeneration
4.6. Nerve Tissue Regeneration
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ding, Q.; Cui, J.; Shen, H.; He, C.; Wang, X.; Shen, S.G.F.; Lin, K. Advances of nanomaterial applications in oral and maxillofacial tissue regeneration and disease treatment. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2020, 13, e1669. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Flint, S.; Moos, K.; Bagan, J. Oral and Maxillofacial Diseases; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- McGue, C.M.; Mañón, V.A.; Viet, C.T. Advances in Tissue Engineering and Implications for Oral and Maxillofacial Reconstruction. J. Calif. Dent. Assoc. 2021, 49, 685–694. [Google Scholar] [PubMed]
- Kawecki, F.; Clafshenkel, W.P.; Fortin, M.; Auger, F.A.; Fradette, J. Biomimetic Tissue-Engineered Bone Substitutes for Maxillofacial and Craniofacial Repair: The Potential of Cell Sheet Technologies. Adv. Healthc. Mater. 2018, 7, e1700919. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, J.; Pan, X.; Ding, Z.; Xie, H.; Wang, X.; Xie, H. Advances of adipose-derived mesenchymal stem cells-based biomaterial scaffolds for oral and maxillofacial tissue engineering. Bioact. Mater. 2021, 6, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, P.; O’Leary, C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, N.; Zandstra, P.W. Engineering cell fitness: Lessons for regenerative medicine. Curr. Opin. Biotechnol. 2017, 47, 7–15. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, D.; Song, C.G.; Kang, P.M. Reactive oxygen species-activated nanomaterials as theranostic agents. Nanomedicine 2015, 10, 2709–2723. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Freschi, M.; Lorenzini, A.; Prata, C.; Maraldi, T.; Hrelia, S. Combination of Epigallocatechin Gallate and Sulforaphane Counteracts In Vitro Oxidative Stress and Delays Stemness Loss of Amniotic Fluid Stem Cells. Oxid. Med. Cell Longev. 2018, 2018, 5263985. [Google Scholar] [CrossRef] [Green Version]
- Cannistrà, M.; Ruggiero, M.; Zullo, A.; Gallelli, G.; Serafini, S.; Maria, M.; Naso, A.; Grande, R.; Serra, R.; Nardo, B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int. J. Surg. 2016, 33 (Suppl. S1), S57–S70. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battula, N.R.; Andreoni, K.A. Oxygenated Preservation Solutions for Organ Preservation. Transplantation 2019, 103, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.S.; Grocott, M.P. Oxygen therapy and anaesthesia: Too much of a good thing? Anaesthesia 2015, 70, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef] [PubMed]
- Mollaee, Z.; Kermani, F.; Moosavi, F.; Kargozar, S.; Vahdati Khaki, J.; Mollazadeh Beidokhti, S. In silico study and experimental evaluation of the solution combustion synthesized manganese oxide (MnO2) nanoparticles. Ceram. Int. 2022, 48, 1659–1672. [Google Scholar] [CrossRef]
- Dutta, D.; Mukherjee, R.; Ghosh, S.; Patra, M.; Mukherjee, M.; Basu, T. Cerium Oxide Nanoparticles as Antioxidant or Pro-oxidant Agents. ACS Appl. Nano Mater. 2022, 5, 1690–1701. [Google Scholar] [CrossRef]
- Ballway, J.W.; Song, B.J. Translational Approaches with Antioxidant Phytochemicals against Alcohol-Mediated Oxidative Stress, Gut Dysbiosis, Intestinal Barrier Dysfunction, and Fatty Liver Disease. Antioxidants 2021, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, N.; Kargozar, S.; Ghayour-Mobarhan, M.; Baino, F.; Pasdar, A.; Sahebkar, A.; Ferns, G.A.A.; Kim, H.W.; Mozafari, M. Curcumin in tissue engineering: A traditional remedy for modern medicine. Biofactors 2019, 45, 135–151. [Google Scholar] [CrossRef]
- Wang, T.; Fan, Q.; Hong, J.; Chen, Z.; Zhou, X.; Zhang, J.; Dai, Y.; Jiang, H.; Gu, Z.; Cheng, Y.; et al. Therapeutic Nanoparticles from Grape Seed for Modulating Oxidative Stress. Small 2021, 17, e2102485. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [Green Version]
- Teti, G.; Salvatore, V.; Ruggeri, A.; Manzoli, L.; Gesi, M.; Orsini, G.; Falconi, M. In vitro reparative dentin: A biochemical and morphological study. Eur. J. Histochem. 2013, 57, e23. [Google Scholar] [CrossRef]
- Baldión, P.A.; Velandia-Romero, M.L.; Castellanos, J.E. Odontoblast-Like Cells Differentiated from Dental Pulp Stem Cells Retain Their Phenotype after Subcultivation. Int. J. Cell Biol. 2018, 2018, 6853189. [Google Scholar] [CrossRef] [Green Version]
- Sajadi-Javan, Z.S.; Varshosaz, J.; Mirian, M.; Manshaei, M.; Aminzadeh, A. Thermo-responsive hydrogels based on methylcellulose/Persian gum loaded with taxifolin enhance bone regeneration: An in vitro/in vivo study. Cellulose 2022, 29, 2413–2433. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Son, K.M.; Park, H.C.; Zhu, T.; Kwon, J.H.; Yang, H.C. Stimulating effects of quercetin and phenamil on differentiation of human dental pulp cells. Eur. J. Oral Sci. 2013, 121, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Huang, G.; Chen, H.; Xu, L.; Qin, S.; Li, A. Research Progress of the Role of Anthocyanins on Bone Regeneration. Front. Pharmacol. 2021, 12, 773660. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.M.; Escobar, J.D.; Bendahan, Z.; Castellanos, J.E. Retinoic and ascorbic acids induce osteoblast differentiation from human dental pulp mesenchymal stem cells. J. Oral Biol. Craniofac. Res. 2021, 11, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Choudhary, S.; Sharma, M.; Kumar, S.S.; Lohan, S.; Bhardwaj, V.; Syan, N.; Jyoti, S. Tea: A native source of antimicrobial agents. Food Res. Int. 2013, 53, 568–584. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Chakraborty, P. Role of Antioxidants in Common Health Diseases. Res. J. Pharm. Technol. 2009, 2, 238–244. [Google Scholar]
- Kharrazi, H.; Vaisi-Raygani, A.; Rahimi, Z.; Tavilani, H.; Aminian, M.; Pourmotabbed, T. Association between enzymatic and non-enzymatic antioxidant defense mechanism with apolipoprotein E genotypes in Alzheimer disease. Clin. Biochem. 2008, 41, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Vovk, M.A.; Batalova, A.A.; Jenkins, R.O.; Goncharov, N.V. The Universal Soldier: Enzymatic and Non-Enzymatic Antioxidant Functions of Serum Albumin. Antioxidants 2020, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Moini, H.; Packer, L.; Saris, N.E. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol. Appl. Pharmacol. 2002, 182, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Becker, B.F.; Reinholz, N.; Leipert, B.; Raschke, P.; Permanetter, B.; Gerlach, E. Role of uric acid as an endogenous radical scavenger and antioxidant. Chest 1991, 100, 176s–181s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoia, M.; Oancea, S. Low-Molecular-Weight Synthetic Antioxidants: Classification, Pharmacological Profile, Effectiveness and Trends. Antioxidants 2022, 11, 638. [Google Scholar] [CrossRef]
- Hermund, D.B. Antioxidant Properties of Seaweed-Derived Substances. In Bioactive Seaweeds for Food Applications; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Sisein, E.A. Biochemistry of Free Radicals and Antioxidants. Sch. Acad. J. Biosci. 2014, 2, 110–118. [Google Scholar]
- Lee, J.; Koo, N.; Min, D.B. Reactive Oxygen Species, Aging, and Antioxidative Nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical: An endogenous toxicant. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Floriano-Sánchez, E.; Villanueva, C.; Medina-Campos, O.N.; Rocha, D.; Sánchez-González, D.J.; Cárdenas-Rodríguez, N.; Pedraza-Chaverrí, J. Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radic. Res. 2006, 40, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Yokozawa, T.; Yamabe, N.; Kim, H.Y.; Park, J.H. ESR study on the structure and hydroxyl radical-scavenging activity relationships of ginsenosides isolated from Panax ginseng C A Meyer. Biol. Pharm. Bull. 2007, 30, 917–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutteridge, J.M. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem. J. 1987, 243, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, Z.Q.; Wu, D. Antioxidative effect of melatonin on DNA and erythrocytes against free-radical-induced oxidation. Chem. Phys. Lipids 2008, 151, 77–84. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Critical Re-Evaluation of DPPH assay: Presence of Pigments Affects the Results. J. Agric. Food Chem. 2019, 67, 7526–7529. [Google Scholar] [CrossRef]
- Shi, H.; Noguchi, N.; Niki, E. Galvinoxyl method for standardizing electron and proton donation activity. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2001; Volume 335, pp. 157–166. [Google Scholar]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [Green Version]
- Üstündaş, M.; Yener, H.B.; Helvaci, Ş.Ş. Parameters affecting lycopene extraction from tomato powder and its antioxidant activity. Anadolu Univ. J. Sci. Technol. A-Appl. Sci. Eng. 2018, 19, 454–467. [Google Scholar] [CrossRef]
- Dong, J.W.; Cai, L.; Xing, Y.; Yu, J.; Ding, Z.T. Re-evaluation of ABTS*+ Assay for Total Antioxidant Capacity of Natural Products. Nat. Prod. Commun. 2015, 10, 2169–2172. [Google Scholar] [CrossRef] [Green Version]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.T.; Shum, J.; Wong, M.; Mikos, A.G.; Young, S. Bone Tissue Engineering Challenges in Oral & Maxillofacial Surgery. Adv. Exp. Med. Biol. 2015, 881, 57–78. [Google Scholar] [CrossRef] [PubMed]
- do Monte, F.A.; Ahuja, N.; Awad, K.R.; Pan, Z.; Young, S.; Kim, H.K.; Aswath, P.; Brotto, M.; Varanasi, V.G. Silicon Oxynitrophosphide Nanoscale Coating Enhances Antioxidant Marker-Induced Angiogenesis During in vivo Cranial Bone-Defect Healing. JBMR Plus 2021, 5, e10425. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Kondo, H.; Nyan, M.; Hao, J.; Miyahara, T.; Ohya, K.; Kasugai, S. Implantation of green tea catechin α-tricalcium phosphate combination enhances bone repair in rat skull defects. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Michaluart, P.; Masferrer, J.L.; Carothers, A.M.; Subbaramaiah, K.; Zweifel, B.S.; Koboldt, C.; Mestre, J.R.; Grunberger, D.; Sacks, P.G.; Tanabe, T.; et al. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999, 59, 2347–2352. [Google Scholar] [PubMed]
- Koltuksuz, U.; Mutuş, H.M.; Kutlu, R.; Ozyurt, H.; Cetin, S.; Karaman, A.; Gürbüz, N.; Akyol, O.; Aydin, N.E. Effects of caffeic acid phenethyl ester and epidermal growth factor on the development of caustic esophageal stricture in rats. J. Pediatr. Surg. 2001, 36, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Kazancioglu, H.O.; Bereket, M.C.; Ezirganli, S.; Aydin, M.S.; Aksakalli, S. Effects of caffeic acid phenethyl ester on wound healing in calvarial defects. Acta Odontol. Scand 2015, 73, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dumanian, Z.P.; Tollemar, V.; Ye, J.; Lu, M.; Zhu, Y.; Liao, J.; Ameer, G.A.; He, T.C.; Reid, R.R. Repair of critical sized cranial defects with BMP9-transduced calvarial cells delivered in a thermoresponsive scaffold. PLoS ONE 2017, 12, e0172327. [Google Scholar] [CrossRef] [Green Version]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food. Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Bhattarai, G.; Jeon, Y.-M.; Choi, K.-C.; Wagle, S.; Sim, H.-J.; Kim, J.-I.; Zhao, S.; Kim, J.-G.; Cho, E.-S.; Kook, S.-H.; et al. Functional improvement of collagen-based bioscaffold to enhance periodontal-defect healing via combination with dietary antioxidant and COMP-angiopoietin 1. Biomater. Adv. 2022, 135, 112673. [Google Scholar] [CrossRef]
- Wang, C.C.; Wang, C.H.; Chen, H.C.; Cherng, J.H.; Chang, S.J.; Wang, Y.W.; Chang, A.; Yeh, J.Z.; Huang, Y.H.; Liu, C.C. Combination of resveratrol-containing collagen with adipose stem cells for craniofacial tissue-engineering applications. Int. Wound J. 2018, 15, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Morochnik, S.; Zhu, Y.; Duan, C.; Cai, M.; Reid, R.R.; He, T.-C.; Koh, J.; Szleifer, I.; Ameer, G.A. A thermoresponsive, citrate-based macromolecule for bone regenerative engineering. J. Biomed. Mater. Res. Part A 2018, 106, 1743–1752. [Google Scholar] [CrossRef]
- Amirthalingam, S.; Lee, S.S.; Rajendran, A.K.; Kim, I.; Hwang, N.S.; Rangasamy, J. Addition of lactoferrin and substance P in a chitin/PLGA-CaSO4 hydrogel for regeneration of calvarial bone defects. Mater. Sci. Eng. C 2021, 126, 112172. [Google Scholar] [CrossRef] [PubMed]
- Altan, B.A.; Kara, I.M.; Nalcaci, R.; Ozan, F.; Erdogan, S.M.; Ozkut, M.M.; Inan, S. Systemic propolis stimulates new bone formation at the expanded suture: A histomorphometric study. Angle Orthod. 2013, 83, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Altan, A.; Yuce, H.; Karataş, Ő.; Taşkan, M.M.; Gevrek, F.; Çolak, S.; Akbulut, N. Free and liposome form of gallic acid improves calvarial bone wound healing in Wistar rats. Asian Pac. J. Trop. Biomed. 2020, 10, 156–163. [Google Scholar] [CrossRef]
- Sasayama, S.; Hara, T.; Tanaka, T.; Honda, Y.; Baba, S. Osteogenesis of Multipotent Progenitor Cells using the Epigallocatechin Gallate-Modified Gelatin Sponge Scaffold in the Rat Congenital Cleft-Jaw Model. Int. J. Mol. Sci. 2018, 19, 3803. [Google Scholar] [CrossRef] [Green Version]
- Ezirganli, S.; Kazancioglu, H.O.; Ozdemir, H.; Inan, D.S.; Tek, M. The Effects of Nigella Sativa Seed Extract on Bone Healing in an Experimental Model. J. Craniofac. Surg. 2016, 27, 1905–1909. [Google Scholar] [CrossRef]
- Chauhan, N.; Gupta, P.; Arora, L.; Pal, D.; Singh, Y. Dexamethasone-loaded, injectable pullulan-poly(ethylene glycol) hydrogels for bone tissue regeneration in chronic inflammatory conditions. Mater. Sci. Eng. C 2021, 130, 112463. [Google Scholar] [CrossRef]
- Laçin, N.; İzol, S.B.; İpek, F.; Tuncer, M.C. Ganoderma lucidum, a promising agent possessing antioxidant and anti-inflammatory effects for treating calvarial defects with graft application in rats. Acta Cir. Bras 2019, 34, e201900904. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Ho, K.-N.; Lee, Y.-C.; Chou, M.-J.; Lew, W.-Z.; Huang, H.-M.; Lai, P.-C.; Feng, S.-W. Melatonin enhances osteogenic differentiation of dental pulp mesenchymal stem cells by regulating MAPK pathways and promotes the efficiency of bone regeneration in calvarial bone defects. Stem Cell Res. Ther. 2022, 13, 73. [Google Scholar] [CrossRef]
- Kim, J.E.; Takanche, J.S.; Yun, B.S.; Yi, H.K. Anti-inflammatory character of Phelligridin D modulates periodontal regeneration in lipopolysaccharide-induced human periodontal ligament cells. J. Periodontal Res. 2018, 53, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Kuo, P.J.; Chin, Y.T.; Weng, I.T.; Lee, H.W.; Huang, H.M.; Lin, H.Y.; Hsiung, C.N.; Chan, Y.H.; Lee, S.Y. Dental Pulp Stem Cell Transplantation with 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside Accelerates Alveolar Bone Regeneration in Rats. J. Endod. 2019, 45, 435–441. [Google Scholar] [CrossRef]
- Wang, R.; Bao, B.; Bao, C.; Wang, S.; Ur Rahman, S.; Hou, C.; Elango, J.; Wu, W. Resveratrol and Celastrol Loaded Collagen Dental Implants Regulate Periodontal Ligament Fibroblast Growth and Osteoclastogenesis of Bone Marrow Macrophages. Chem. Biodivers. 2020, 17, e2000295. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Anti-inflammatory, anti-osteoclastic, and antioxidant activities of genistein protect against alveolar bone loss and periodontal tissue degradation in a mouse model of periodontitis. J. Biomed. Mater. Res. A 2017, 105, 2510–2521. [Google Scholar] [CrossRef] [PubMed]
- Thahir, H.; Irawaty Djais, A.; Nasir, M.; Rahayu Feblina, A.; Annisa, A.; Etriyani, N.; Achmad, H. Virgin Coconut Oil as a New Concept for Periodontal Tissue Regeneration via Expressions of TNF-α and TGF-β1. Int. J. Biomater. 2022, 2022, 7562608. [Google Scholar] [CrossRef] [PubMed]
- Gültekin, S.E.; Sengüven, B.; Sofuoğlu, A.; Taner, L.; Koch, M. Effect of the topical use of the antioxidant taurine on the two basement membrane proteins of regenerating oral gingival epithelium. J. Periodontol. 2012, 83, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ye, A. Salvadora persica Extract-laden Jellyfish Collagen Hybrid Constructs for Periodontal Tissue Regeneration. J. Turk. Chem. Soc. Sect. A Chem. 2019, 6, 51–62. [Google Scholar]
- Alvarez Echazú, M.I.; Olivetti, C.E.; Peralta, I.; Alonso, M.R.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development of pH-responsive biopolymer-silica composites loaded with Larrea divaricata Cav. extract with antioxidant activity. Colloids Surf. B Biointerfaces 2018, 169, 82–91. [Google Scholar] [CrossRef]
- Shaheen, M.A.; Elmeadawy, S.H.; Bazeed, F.B.; Anees, M.M.; Saleh, N.M. Innovative coenzyme Q(10)-loaded nanoformulation as an adjunct approach for the management of moderate periodontitis: Preparation, evaluation, and clinical study. Drug Deliv. Transl. Res. 2020, 10, 548–564. [Google Scholar] [CrossRef]
- Saita, M.; Kaneko, J.; Sato, T.; Takahashi, S.S.; Wada-Takahashi, S.; Kawamata, R.; Sakurai, T.; Lee, M.C.; Hamada, N.; Kimoto, K.; et al. Novel antioxidative nanotherapeutics in a rat periodontitis model: Reactive oxygen species scavenging by redox injectable gel suppresses alveolar bone resorption. Biomaterials 2016, 76, 292–301. [Google Scholar] [CrossRef]
- Manconi, M.; Petretto, G.; D’Hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus essential oil extraction, characterization and incorporation in phospholipid vesicles for the antioxidant/antibacterial treatment of oral cavity diseases. Colloids Surf. B Biointerfaces 2018, 171, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Celiksoy, V.; Moses, R.L.; Sloan, A.J.; Moseley, R.; Heard, C.M. Evaluation of the In Vitro Oral Wound Healing Effects of Pomegranate (Punica granatum) Rind Extract and Punicalagin, in Combination with Zn (II). Biomolecules 2020, 10, 1234. [Google Scholar] [CrossRef]
- Babaee, N.; Zabihi, E.; Mohseni, S.; Moghadamnia, A.A. Evaluation of the therapeutic effects of Aloe vera gel on minor recurrent aphthous stomatitis. Dent. Res. J. 2012, 9, 381–385. [Google Scholar]
- Kılıç, C.; Güleç Peker, E.G.; Acartürk, F.; Kılıçaslan, S.M.; Çoşkun Cevher, Ş. Investigation of the effects of local glutathione and chitosan administration on incisional oral mucosal wound healing in rabbits. Colloids Surf. B Biointerfaces 2013, 112, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Hakim, R.F.; Fakhrurrazi, F.; Dinni, D. Effect of Carica papaya Extract toward Incised Wound Healing Process in Mice (Mus musculus) Clinically and Histologically. Evid. Based Complement. Alternat. Med. 2019, 2019, 8306519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salari Sedigh, S.; Mozafari, A.; Sadeghi, M.; Eslammanesh, T.; Fatemi, I. Oral Wound Healing Activities of Pistacia vera Hydroalcoholic Extract. Pist. Health J. 2022, 5, 3–10. [Google Scholar] [CrossRef]
- Ghodoosi, S. Efficacy of 10% Calendula officinalis extract gel for oral wound healing in rats. J. Biol. Stud. 2022, 5, 52–63. [Google Scholar]
- Sinjari, B.; Pizzicannella, J.; D’Aurora, M.; Zappacosta, R.; Gatta, V.; Fontana, A.; Trubiani, O.; Diomede, F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-inflammatory Activities via NFkB/ERK/pERK Pathway in Human Dental Pulp Treated With 2-HydroxyEthyl MethAcrylate (HEMA). Front. Physiol. 2019, 10, 633. [Google Scholar] [CrossRef]
- Jun, S.-K.; Yoon, J.-Y.; Mahapatra, C.; Park, J.H.; Kim, H.-W.; Kim, H.-R.; Lee, J.-H.; Lee, H.-H. Ceria-incorporated MTA for accelerating odontoblastic differentiation via ROS downregulation. Dent. Mater. 2019, 35, 1291–1299. [Google Scholar] [CrossRef]
- Chang, J.-H.; Kim, D.-W.; Kim, S.-G.; Kim, T.-W. Alleviation of Oxidative Stress in Dental Pulp Cells Following 4-Hexylresorcinol Administration in a Rat Model. Appl. Sci. 2021, 11, 3637. [Google Scholar] [CrossRef]
- Leite, M.F.; Lima, A.M.; Otton, R. Combination of astaxanthin and fish oil supplementation alters antioxidant enzyme profile of dental pulp tissue. Int. Endod. J. 2012, 45, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, H.; Yamada, M.; Deyama, Y.; Suzuki, K.; Kaga, M.; Yawaka, Y.; Ogawa, T. Effect of N-acetylcysteine on rat dental pulp cells cultured on mineral trioxide aggregate. J. Endod. 2011, 37, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Sairaman, S.; Nivedhitha, M.S.; Shrivastava, D.; Al Onazi, M.A.; Algarni, H.A.; Mustafa, M.; Alqahtani, A.R.; AlQahtani, N.; Teja, K.V.; Janani, K.; et al. Biocompatibility and antioxidant activity of a novel carrageenan based injectable hydrogel scaffold incorporated with Cissus quadrangularis: An in vitro study. BMC Oral Health 2022, 22, 377. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Burdis, R.; Kelly, D.J. Printing New Bones: From Print-and-Implant Devices to Bioprinted Bone Organ Precursors. Trends Mol. Med. 2021, 27, 700–711. [Google Scholar] [CrossRef]
- Vargas Fda, S.; Soares, D.G.; Basso, F.G.; Hebling, J.; Costa, C.A. Dose-response and time-course of α-tocoferol mediating the cytoprotection of dental pulp cells against hydrogen peroxide. Braz. Dent. J. 2014, 25, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Abedi, N.; Rajabi, N.; Kharaziha, M.; Nejatidanesh, F.; Tayebi, L. Layered scaffolds in periodontal regeneration. J. Oral Biol. Craniofac. Res. 2022, 12, 782–797. [Google Scholar] [CrossRef]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef]
- Fakheran, O.; Khademi, A.; Bagherniya, M.; Sathyapalan, T.; Sahebkar, A. The Effects of Nutraceuticals and Bioactive Natural Compounds on Chronic Periodontitis: A Clinical Review. Adv. Exp. Med. Biol. 2021, 1328, 59–80. [Google Scholar] [CrossRef]
- Cutando, A.; Gómez-Moreno, G.; Arana, C.; Acuña-Castroviejo, D.; Reiter, R.J. Melatonin: Potential functions in the oral cavity. J. Periodontol. 2007, 78, 1094–1102. [Google Scholar] [CrossRef]
- Solá, V.M.; Aguilar, J.J.; Farías, A.; Vazquez Mosquera, A.P.; Peralta López, M.E.; Carpentieri, A.R. Melatonin protects gingival mesenchymal stem cells and promotes differentiation into osteoblasts. Cell Biochem. Funct. 2022, 40, 636–646. [Google Scholar] [CrossRef]
- Zohery, A.A.; Meshri, S.M.; Madi, M.I.; Abd El Rehim, S.S.; Nour, Z.M. Egyptian propolis compared to nanohydroxyapatite graft in the treatment of Class II furcation defects in dogs. J. Periodontol. 2018, 89, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Arpornmaeklong, P.; Sareethammanuwat, M.; Apinyauppatham, K.; Boonyuen, S. Characteristics and biologic effects of thermosensitive quercetin-chitosan/collagen hydrogel on human periodontal ligament stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1656–1670. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, D.; Li, Q.; Sun, X.; Song, Y.; Wang, C. Curcumin inhibits inflammatory response and bone loss during experimental periodontitis in rats. Acta Odontol. Scand. 2013, 71, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Hu, B.B.; Shi, X.L.; Ren, M.M.; Yu, W.B.; Cen, S.D.; Hu, R.D.; Deng, H. Baicalein enhances the osteogenic differentiation of human periodontal ligament cells by activating the Wnt/β-catenin signaling pathway. Arch. Oral Biol. 2017, 78, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, A.; Kunimatsu, R.; Yoshimi, Y.; Tsuka, Y.; Awada, T.; Horie, K.; Gunji, H.; Abe, T.; Nakajima, K.; Kitagawa, M.; et al. Baicalin Promotes Osteogenic Differentiation of Human Cementoblast Lineage Cells Via the Wnt/β Catenin Signaling Pathway. Curr. Pharm. Des. 2018, 24, 3980–3987. [Google Scholar] [CrossRef] [PubMed]
- Kresnoadi, U.; Ariani, M.D.; Djulaeha, E.; Hendrijantini, N. The potential of mangosteen (Garcinia mangostana) peel extract, combined with demineralized freeze-dried bovine bone xenograft, to reduce ridge resorption and alveolar bone regeneration in preserving the tooth extraction socket. J. Indian Prosthodont. Soc. 2017, 17, 282–288. [Google Scholar] [CrossRef]
- EI behairy, R.A.A.; Hammad, H.G.H.; Ahmed, I.H.; Khafagi, M.G. Evaluation of the effect of hyaluronic acid and chitosan biocomposite natural polymers in alveolar ridge preservation: An experimental study in dogs. Egypt. Dent. J. 2019, 65, 2171–2181. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Xiong, Y.; Zhang, W.; Ma, X.; Xu, X. Metformin promotes osteogenic differentiation and protects against oxidative stress-induced damage in periodontal ligament stem cells via activation of the Akt/Nrf2 signaling pathway. Exp. Cell Res. 2020, 386, 111717. [Google Scholar] [CrossRef]
- Kibar, H.; Arslan, Y.E.; Ceylan, A.; Karaca, B.; Haliscelik, O.; Kiran, F. Weissella cibaria EIR/P2-derived exopolysaccharide: A novel alternative to conventional biomaterials targeting periodontal regeneration. Int. J. Biol. Macromol. 2020, 165, 2900–2908. [Google Scholar] [CrossRef]
- Liu, J.; Huang, F.; He, H.W. Melatonin effects on hard tissues: Bone and tooth. Int. J. Mol. Sci. 2013, 14, 10063–10074. [Google Scholar] [CrossRef] [Green Version]
- Giuca, M.R.; Lardani, L.; Ligori, S.; Carli, E.; Giuca, G.; Miceli, M. Oral manifestations in paediatric patients with hepatobiliary diseases: A review. J. Biol. Regul. Homeost. Agents 2021, 35, 117–125. [Google Scholar] [CrossRef]
- Fu, Z.; Zhuang, Y.; Cui, J.; Sheng, R.; Tomás, H.; Rodrigues, J.; Zhao, B.; Wang, X.; Lin, K. Development and challenges of cells- and materials-based tooth regeneration. Eng. Regen. 2022, 3, 163–181. [Google Scholar] [CrossRef]
- Giacaman, R.A.; Contzen, M.P.; Yuri, J.A.; Muñoz-Sandoval, C. Anticaries effect of an antioxidant-rich apple concentrate on enamel in an experimental biofilm-demineralization model. J. Appl. Microbiol. 2014, 117, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Basir, L.; Kalhori, S.; Zare Javid, A.; Khaneh Masjedi, M. Anticaries Activity of Curcumin on Decay Process in Human Tooth Enamel Samples (In Vitro Study). J. Natl. Med. Assoc. 2018, 110, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Murata, R.M.; Duarte, S. Antimicrobial traits of tea- and cranberry-derived polyphenols against Streptococcus mutans. Caries Res. 2011, 45, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Tsuji, M.; Okuda, J.; Sasaki, H.; Nakano, K.; Osawa, K.; Shimura, S.; Ooshima, T. Inhibitory effects of cacao bean husk extract on plaque formation in vitro and in vivo. Eur. J. Oral Sci. 2004, 112, 249–252. [Google Scholar] [CrossRef]
- Kharouf, N.; Haikel, Y.; Ball, V. Polyphenols in Dental Applications. Bioengineering 2020, 7, 72. [Google Scholar] [CrossRef]
- Olaru, M.; Sachelarie, L.; Calin, G. Hard Dental Tissues Regeneration-Approaches and Challenges. Materials 2021, 14, 2558. [Google Scholar] [CrossRef]
- van Strijp, A.J.; Takatsuka, T.; Sono, R.; Iijima, Y. Inhibition of dentine collagen degradation by hesperidin: An in situ study. Eur. J. Oral Sci. 2015, 123, 447–452. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, Y.; Fang, X.; Yang, J.; Chen, Z. Epigallocatechin-3-Gallate Promotes Osteo-/Odontogenic Differentiation of Stem Cells from the Apical Papilla through Activating the BMP-Smad Signaling Pathway. Molecules 2021, 26, 1580. [Google Scholar] [CrossRef]
- Chen, H.; Kang, J.; Zhang, F.; Yan, T.; Fan, W.; He, H.; Huang, F. SIRT4 regulates rat dental papilla cell differentiation by promoting mitochondrial functions. Int. J. Biochem. Cell Biol. 2021, 134, 105962. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.C.; Venkatesh, K.V.; Nandini, V.; Sihivahanan, D.; Alamoudi, A.; Bahammam, H.A.; Bahammam, S.A.; Zidane, B.; Bahammam, M.A.; Chohan, H.; et al. Evaluating the Effect of Tideglusib-Loaded Bioactive Glass Nanoparticles as a Potential Dentine Regenerative Material. Materials 2022, 15, 4567. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, H.; Yamada, M.; Iwasa, F.; Ueno, T.; Deyama, Y.; Suzuki, K.; Yawaka, Y.; Ogawa, T. Amino acid derivative-mediated detoxification and functionalization of dual cure dental restorative material for dental pulp cell mineralization. Biomaterials 2010, 31, 7213–7225. [Google Scholar] [CrossRef] [PubMed]
- Salmon, C.R.; Tomazela, D.M.; Ruiz, K.G.; Foster, B.L.; Paes Leme, A.F.; Sallum, E.A.; Somerman, M.J.; Nociti, F.H., Jr. Proteomic analysis of human dental cementum and alveolar bone. J. Proteom. 2013, 91, 544–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, P.; Yu, Z.; Tran, Q.T.; Bhatti, F.U.; Zhu, X.; Huang, G.T. Cementogenic genes in human periodontal ligament stem cells are downregulated in response to osteogenic stimulation while upregulated by vitamin C treatment. Cell Tissue Res. 2017, 368, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Leon, E.R.; Iwasaki, K.; Komaki, M.; Kojima, T.; Ishikawa, I. Osteogenic effect of interleukin-11 and synergism with ascorbic acid in human periodontal ligament cells. J. Periodontal. Res. 2007, 42, 527–535. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Garg, V.; Raju, A.; Singh, P. Adjunctive Local Delivery of Aloe Vera Gel in Patients With Type 2 Diabetes and Chronic Periodontitis: A Randomized, Controlled Clinical Trial. J. Periodontol. 2016, 87, 268–274. [Google Scholar] [CrossRef]
- Takeda, K.; Mizutani, K.; Matsuura, T.; Kido, D.; Mikami, R.; Buranasin, P.; Saito, N.; Kominato, H.; Takemura, S.; Nakagawa, K.; et al. Antioxidant effect of enamel matrix derivative for early phase of periodontal tissue regeneration in diabetes. J. Periodontol. 2022, 93, 1206–1217. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. Bras Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Huang, Y.; Qiu, Z.; Xu, Z.; Li, D.; Chen, L.; Jiang, J.; Gao, L. Vitamin B(2) functionalized iron oxide nanozymes for mouth ulcer healing. Sci. China Life Sci. 2020, 63, 68–79. [Google Scholar] [CrossRef]
- Krismaya, I.D.G. Effects of lime (Citrus aurantifolia christm. swingle) peel extract on fibroblast proliferation and angiogenesis in rat’s tooth extraction sockets. Biochem. Cell. Arch. 2019, 19, 4917–4919. [Google Scholar]
- Fidoski, J.; Benedetti, A.; Kirkov, A.; Iliev, A.; Stamatoski, A.; Baftijari, D. Nano-emulsion complex (propolis and vitamin C) promotes wound healing in the oral mucosa. Oral Maxillofac. Pathol. J. 2020, 11, 1–5. [Google Scholar]
- Shah, D.; Lynd, T.; Ho, D.; Chen, J.; Vines, J.; Jung, H.D.; Kim, J.H.; Zhang, P.; Wu, H.; Jun, H.W.; et al. Pulp-Dentin Tissue Healing Response: A Discussion of Current Biomedical Approaches. J. Clin. Med. 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, M.F.; De Lima, A.; Massuyama, M.M.; Otton, R. In vivo astaxanthin treatment partially prevents antioxidant alterations in dental pulp from alloxan-induced diabetic rats. Int. Endod. J. 2010, 43, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Delle Monache, S.; Pulcini, F.; Frosini, R.; Mattei, V.; Talesa, V.N.; Antognelli, C. Methylglyoxal-Dependent Glycative Stress Is Prevented by the Natural Antioxidant Oleuropein in Human Dental Pulp Stem Cells through Nrf2/Glo1 Pathway. Antioxidants 2021, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Ebrahimpour, S.; Nourbakhsh, N.; Talebi, S.; Esmaeili, A. Protective effect of quercetin on alteration of antioxidant genes expression and histological changes in the dental pulp of the streptozotocin-diabetic rats. Arch. Oral Biol. 2021, 125, 105088. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Ahn, S.G.; Kim, S.A. Cinnamaldehyde protects human dental pulp cells against oxidative stress through the Nrf2/HO-1-dependent antioxidant response. Eur. J. Pharmacol. 2017, 815, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Sanchez, P.K.V.; Fernandes, R.R.; Souza, F.P.P.; Siéssere, S.; Bombonato-Prado, K.F. Effect of grape seed extract (GSE) on functional activity and mineralization of OD-21 and MDPC-23 cell lines. Braz. Oral Res. 2019, 33, e013. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Marconi, G.D.; Guarnieri, S.; D’Attilio, M.; Cavalcanti, M.; Mariggiò, M.A.; Pizzicannella, J.; Trubiani, O. A Novel Role of Ascorbic Acid in Anti-Inflammatory Pathway and ROS Generation in HEMA Treated Dental Pulp Stem Cells. Materials 2019, 13, 130. [Google Scholar] [CrossRef] [Green Version]
- Yari, D.; Ebrahimzadeh, M.H.; Movaffagh, J.; Shahroodi, A.; Shirzad, M.; Qujeq, D.; Moradi, A. Biochemical Aspects of Scaffolds for Cartilage Tissue Engineering; from Basic Science to Regenerative Medicine. Arch. Bone Jt. Surg. 2022, 10, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Chen, Y.; Hashim, R.; He, C.; Mo, X.; Zhou, X. Reactive Oxygen Species-Based Biomaterials for Regenerative Medicine and Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2021, 9, 821288. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.K.; Samson, S.E. Benefits of antioxidant supplements for knee osteoarthritis: Rationale and reality. Nutr. J. 2016, 15, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, K.Y.; Ima-Nirwana, S. The Role of Vitamin E in Preventing and Treating Osteoarthritis—A Review of the Current Evidence. Front. Pharmacol. 2018, 9, 946. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Shao, X.; Ling, P.; Liu, F.; Han, G.; Wang, F. Recent advances in polysaccharides for osteoarthritis therapy. Eur. J. Med. Chem. 2017, 139, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Yang, X.; Jiang, X.; Kumar, A.; Long, H.; Xie, J.; Zheng, L.; Zhao, J. Dopamine-melanin nanoparticles scavenge reactive oxygen and nitrogen species and activate autophagy for osteoarthritis therapy. Nanoscale 2019, 11, 11605–11616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, W.; Shafiq, M.; Tang, J.; Hao, J.; Xie, X.; Yuan, Z.; Xiao, X.; Liu, Y.; Mo, X. Three-dimensional porous gas-foamed electrospun nanofiber scaffold for cartilage regeneration. J. Colloid Interface Sci. 2021, 603, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Cui, F.; Du, X.; Shang, G.; Xiao, W.; Yang, X.; Cui, Q. Antioxidative nanofullerol inhibits macrophage activation and development of osteoarthritis in rats. Int. J. Nanomed. 2019, 14, 4145–4155. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.Y.; Li, W.; Xu, Y.; Jin, E.H.; Tu, Y.Y. Evaluation of the antioxidant effects of four main theaflavin derivatives through chemiluminescence and DNA damage analyses. J. Zhejiang Univ. Sci. B 2011, 12, 744–751. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zheng, J. Theaflavins prevent cartilage degeneration via AKT/FOXO3 signaling in vitro. Mol. Med. Rep. 2019, 19, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Woo, M.; Kwon, D.H.; Choi, Y.H.; Noh, J.S. Inhibitory effects of skate cartilage chondroitin sulfate-rich extract on the production of inflammatory mediators and ROS in lipopolysaccharide-treated murine macrophages: A comparison with shark cartilage chondroitin sulfate. In Vitro Cell Dev. Biol. Anim. 2020, 56, 271–276. [Google Scholar] [CrossRef]
- Liang, R.; Zhao, J.; Li, B.; Cai, P.; Loh, X.J.; Xu, C.; Chen, P.; Kai, D.; Zheng, L. Implantable and degradable antioxidant poly(ε-caprolactone)-lignin nanofiber membrane for effective osteoarthritis treatment. Biomaterials 2020, 230, 119601. [Google Scholar] [CrossRef]
- Liang, R.; Yang, X.; Yew, P.Y.M.; Sugiarto, S.; Zhu, Q.; Zhao, J.; Loh, X.J.; Zheng, L.; Kai, D. PLA-lignin nanofibers as antioxidant biomaterials for cartilage regeneration and osteoarthritis treatment. J. Nanobiotechnol. 2022, 20, 327. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, L.; Xu, X.; Fan, Y.; Xue, X.; Shen, M.; Shi, X. Targeted Combination of Antioxidative and Anti-Inflammatory Therapy of Rheumatoid Arthritis using Multifunctional Dendrimer-Entrapped Gold Nanoparticles as a Platform. Small 2020, 16, e2005661. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Yan, J.; Zhou, W.; Gao, S.; Liu, S.; Li, Q.; Zheng, Y.; Cheng, Y.; Guo, Q. Tannic acid/Sr(2+)-coated silk/graphene oxide-based meniscus scaffold with anti-inflammatory and anti-ROS functions for cartilage protection and delaying osteoarthritis. Acta Biomater. 2021, 126, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Sintra, T.E.; Luís, A.; Rocha, S.N.; Lobo Ferreira, A.I.; Gonçalves, F.; Santos, L.M.; Neves, B.M.; Freire, M.G.; Ventura, S.P.; Coutinho, J.A. Enhancing the antioxidant characteristics of phenolic acids by their conversion into cholinium salts. ACS Sustain. Chem. Eng. 2015, 3, 2558–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, S.; Zhou, X.; Sang, W.; Wang, C.; Lu, H.; Xu, Y.; Zhong, Y.; Zhu, L.; He, C.; Ma, J. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact. Mater. 2021, 6, 2372–2389. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ding, J.; Xu, P.; Feng, X.; Wang, Z.; Zhou, T.; Tu, C.; Cao, W.; Xie, J.; Deng, L.; et al. A cell-free ROS-responsive hydrogel/oriented poly(lactide-co-glycolide) hybrid scaffold for reducing inflammation and restoring full-thickness cartilage defectsin vivo. Biomed. Mater. 2021, 16, 064101. [Google Scholar] [CrossRef]
- Naujoks, C.; Meyer, U.; Wiesmann, H.P.; Jäsche-Meyer, J.; Hohoff, A.; Depprich, R.; Handschel, J. Principles of cartilage tissue engineering in TMJ reconstruction. Head Face Med. 2008, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Stegenga, B. Nomenclature and classification of temporomandibular joint disorders. J. Oral Rehabil. 2010, 37, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Pagano, S.; Lombardo, G.; Caponi, S.; Costanzi, E.; Di Michele, A.; Bruscoli, S.; Xhimitiku, I.; Coniglio, M.; Valenti, C.; Mattarelli, M.; et al. Bio-mechanical characterization of a CAD/CAM PMMA resin for digital removable prostheses. Dent. Mater. 2021, 37, e118–e130. [Google Scholar] [CrossRef]
- Braz, M.A.; Freitas Portella, F.; Seehaber, K.A.; Bavaresco, C.S.; Rivaldo, E.G. Association between oxidative stress and temporomandibular joint dysfunction: A narrative review. J. Oral Rehabil. 2020, 47, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Yamada, M.; Sugita, Y.; Ogawa, T. N-acetyl cysteine protects TMJ chondrocytes from oxidative stress. J. Dent. Res. 2011, 90, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Arai, Y.; Mazda, O.; Kishida, T.; Takahashi, K.A.; Sakao, K.; Saito, M.; Honjo, K.; Imanishi, J.; Kubo, T. N-acetylcysteine prevents nitric oxide-induced chondrocyte apoptosis and cartilage degeneration in an experimental model of osteoarthritis. J. Orthop. Res. 2010, 28, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones 2020, 25, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Izawa, T.; Hutami, I.R.; Tanaka, E. Potential Role of Rebamipide in Osteoclast Differentiation and Mandibular Condylar Cartilage Homeostasis. Curr. Rheumatol. Rev. 2018, 14, 62–69. [Google Scholar] [CrossRef]
- da Costa, B.R.; Reichenbach, S.; Keller, N.; Nartey, L.; Wandel, S.; Jüni, P.; Trelle, S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet 2017, 390, e21–e33. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, S.; Chen, X.; Shi, J. The Antioxidant Resveratrol Protects against Chondrocyte Apoptosis by Regulating the COX-2/NF-κB Pathway in Created Temporomandibular Osteoarthritis. Biomed. Res. Int. 2021, 2021, 9978651. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Sultan, M.T.; Kim, S.H.; Kumar, V.; Yeon, Y.K.; Lee, O.J.; Park, C.H. Artificial Auricular Cartilage Using Silk Fibroin and Polyvinyl Alcohol Hydrogel. Int. J. Mol. Sci. 2017, 18, 1707. [Google Scholar] [CrossRef] [Green Version]
- Dashnyam, K.; Lee, J.H.; Mandakhbayar, N.; Jin, G.Z.; Lee, H.H.; Kim, H.W. Intra-articular biomaterials-assisted delivery to treat temporomandibular joint disorders. J. Tissue Eng. 2018, 9, 2041731418776514. [Google Scholar] [CrossRef] [Green Version]
- Bhamare, N.C.; Tardalkar, K.R.; Kshersagar, J.; Desai, S.R.; Marsale, T.B.; Nimbalkar, M.S.; Sharma, S.; Joshi, M.G. Tissue engineered human ear pinna derived from decellularized goat ear cartilage: Clinically useful and biocompatible auricle construct. Cell Tissue Bank 2022, 23, 43–55. [Google Scholar] [CrossRef]
- Qi, H.L.; Chen, Z.Y.; Qin, Y.H.; Li, Y.Z. Curcumin ameliorates H(2)O(2)-induced inflammatory response in chondrocytes by inducing autophagy activation. Exp. Ther. Med. 2022, 23, 272. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.A.; Wall, M.P.; Greinwald, J.H., Jr. Effects of dimethylthiourea, melatonin, and hyperbaric oxygen therapy on the survival of reimplanted rabbit auricular composite grafts. Otolaryngol. Head Neck Surg. 1999, 121, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Çakan, D.; Aydın, S.; Demir, G.; Başak, K. The effect of curcumin on healing in an animal nasal septal perforation model. Laryngoscope 2019, 129, E349–E354. [Google Scholar] [CrossRef] [PubMed]
- Yanilmaz, M.; Akduman, D.; Sagun, Ö.F.; Haksever, M.; Yazicilar, O.; Orhan, I.; Akpolat, N.; Gök, U. The effects of aminoguanidine, methylprednisolone, and melatonin on nerve recovery in peripheral facial nerve neurorrhaphy. J. Craniofac. Surg. 2015, 26, 667–672. [Google Scholar] [CrossRef]
- Samara, G.J.; Schaffner, A.D.; Eisenstat, J.; Nguyen, H.L. The effects of the plasminogen pathway on scar tissue formation. Laryngoscope 2004, 114, 46–49. [Google Scholar] [CrossRef]
- Ito, M.; Ohbayashi, M.; Furukawa, M.; Okoyama, S. Neuroprotective effects of TJ-23 (Tokishakuyakusan) on adult rat motoneurons following peripheral facial nerve axotomy. Otolaryngol. Head Neck Surg. 2007, 136, 225–230. [Google Scholar] [CrossRef]
- Henderson, J.T.; Javaheri, M.; Kopko, S.; Roder, J.C. Reduction of Lower Motor Neuron Degeneration inwobbler Mice byN-Acetyl-L-Cysteine. J. Neurosci. 1996, 16, 7574. [Google Scholar] [CrossRef] [Green Version]
- Hoshida, S.; Hatano, M.; Furukawa, M.; Ito, M. Neuroprotective effects of vitamin E on adult rat motor neurones following facial nerve avulsion. Acta Oto-Laryngol. 2009, 129, 330–336. [Google Scholar] [CrossRef]
- Salomone, R.; Jácomo, A.L.; Nascimento, S.B.D.; Lezirovitz, K.; Hojaij, F.C.; Costa, H.; Bento, R.F. Polyethylene glycol fusion associated with antioxidants: A new promise in the treatment of traumatic facial paralysis. Head Neck 2018, 40, 1489–1497. [Google Scholar] [CrossRef]
- Sereflican, M.; Yurttas, V.; Ozyalvacli, G.; Terzi, E.H.; Turkoglu, S.A.; Yildiz, S.; Ilgaz, Y.; Seyhan, S.; Oral, M.; Dagli, M. The histopathological and electrophysiological effects of thymoquinone and methylprednisolone in a rabbit traumatic facial nerve paralysis model. Am. J. Otolaryngol. 2016, 37, 407–415. [Google Scholar] [CrossRef]
- Zucki, F.; Morata, T.C.; Duarte, J.L.; Ferreira, M.C.F.; Salgado, M.H.; Alvarenga, K.F. The maturation state of the auditory nerve and brainstem in rats exposed to lead acetate and supplemented with ferrous sulfate. Braz. J. Otorhinolaryngol. 2018, 84, 150–158. [Google Scholar] [CrossRef] [PubMed]







| Antioxidant Material | Natural/Synthetic | Form/Dose | In Vitro/In Vivo and Targeted Region | Significant Results | Ref | |
|---|---|---|---|---|---|---|
| Craniofacial bone | Polyethyleneglycol citrate-co-N-isopropylacry lamid | Synthetic | Scaffold | Human mesenchymal stem cells (hMSCs)/a mouse model with critical defects | Increased osteogenic differentiation of hMSCs/ robust mineralization and osteogenesis in the animal model (Figure 2B) | [65] |
| Propolis | Natural | Systemic application (100 mg/kg) | Premaxillary region in rats | Enhanced bone remodeling and formation of new capillaries | [67] | |
| Gallic acid | Natural | Powder form and liposome form | Calvarial defects of Wistar rats | Formation of new bone | [68] | |
| Epigallocatechin gallate | Natural | Gel form | Congenital cleft-jaw model (in vivo) | Increased osteogenic properties and bone regeneration | [69] | |
| Nigella sativa (N. Sativa) | Natural | Systemically applied with gelatin sponge graft | Calvarial rat defects | New bone tissue formation | [70] | |
| Oxidized pullulan | Synthetic | Injectable hydrogel | Murine osteoblast precursor cells (MC3T3-E1) | Reduced inflammatory markers, such as IL-6 and IL-1, enhanced osteogenesis | [71] | |
| Lactoferrin and substance P | Synthetic | Injectable hydrogel | Critical-sized calvarial defects in mice | Enhanced bone defect healing (Figure 2C) | [66] | |
| Ganoderma lucidum, a kind of mushroom | Natural | Systemic application | Calvarial rat defects | Osteonectin production | [72] | |
| Melatonin | Natural | Local application | Dental pulp stem cell (DPSCs)/rat calvarial defects | Promoted proliferation and osteogenic differentiation of DPSCs by regulating COX-2/NF-κB/p38/ERK MARK. | [73] | |
| Periodontal tissue | Phelligridin D | Natural | Local application | Human periodontal ligament cell (HPDLCs) induced by glucose oxidative stress | Enhanced osteogenic and cementogenic properties of HPDLCs | [74] |
| 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside | Natural | Local application | Human dental pulp stem cells | Increased osteogenic differentiation of hDPSCs and Improved bone formation | [75] | |
| Resveratrol and celastrol | Natural | Collagen film | Human periodontal ligament fibroblast cells | Increased proliferation of HPDLF cells | [76] | |
| Genistein | Natural | Systemically intraperitoneal injected | Mice model /human gingival fibroblasts (hGFs) | Protecting hGFs from ROS products, preventing osteoclast differentiation | [77] | |
| Virgin coconut oil | Natural | Gel form | Wistar rats | Promoted periodontal tissue healing by effecting the level of inflammatory markers, includingTNF-α and TGF-β1 | [78] | |
| Taurine | Natural | Collagen membrane | Gingival epithelium | Rapid reepithelization in gingiva | [79] | |
| Salvadora persica/jellyfish collagen | Natural | Scaffolds | hPDLF cells | Enhanced cell proliferation and regeneration | [80] | |
| Aqueous Larrea divaricata Cav | Natural | Scaffolds | 3T3 fibroblasts cells | Increased fibroblast proliferation | [81] | |
| Coenzyme Q10 | Natural | Nanoparticles | In vitro and in vivo in human | Enhanced antioxidant capacity of periodontium, facilitated chronic periodontitis treatment | [82] | |
| Flower micelles | Natural | Injectable gel | In vitro/in vivo in Sprague–Dawley rats with periodontitis | Inhibiting P. gingivalis-induced bone loss | [83] | |
| Wound healing | Thymus essential oil | Natural | Phospholipid vesicles | Keratinocytes cells | Enhanced antioxidant and antibacterial effects on damaged oral tissue | [84] |
| Pomegranate ingredient (punicalagin) + Zn | Natural | Solution | Human gingival fibroblast | Induced antioxidant effect, enhanced human fibroblast proliferation, and healing of gingiva injury | [85] | |
| Aloe vera (AV) | Natural | Gel form | Minor aphthous lesions | Decreased lesion pain, wound size, and also period of wound healing | [86] | |
| Glutathione and chitosan | Natural | Local application | Intraoral incision of rabbits | Regeneration of oral soft tissue | [87] | |
| Papaya | Natural | Local application | Labial injuries in mandible anterior parts in mice | Increased healing process in oral ulcers in mice, perfect epithelial layer formation and fibrillation | [88] | |
| Hydroalcoholic extract of pistachio vera seeds(PSE) | Natural | Local application | Rat tongue injuries | Enhanced healing of oral wounds/increased fibroblast proliferation | [89] | |
| Calendula officinalis (C. officinalis) | Natural | Local application | Injuries in buccal mucosa in Wistar rats | Improved wound healing | [90] | |
| Curcumin | Natural | Liposomal formulation | Dental pulp stem cells | Increased cell proliferation/inhibition of inflammatory cytokines released through the NFkB/ERK and pERK signaling cascades | [91] | |
| Ceria nanoparticles | Synthetic | Nanoparticle form | hCPSCs cells | Improved odontoblastic differentiation of hDPSCs, reduced high levels of intracellular ROS in hDPSCs | [92] | |
| 4-Hexylresorcinol | Natural | Ointment form | Dental pulp cells/in vivo test in rat model | Reduced inflammatory cytokines, such as TNF-α and IL-1β, and increased antioxidant capacities and activities | [93] | |
| Astaxanthin /Fish oil | Natural | Systemic application | In vivo rat model | Keeping pulp tissue safe from oxidative stress-related diseases | [94] | |
| Amino acid, N-acetylcysteine | Natural | Local application (form of powder and liquid) | Rat dental pulp cell extract | Increased cell proliferation and attachment, improved cellular redox system | [95] | |
| Carrageenan + Cossus quadrangularis, a natural agent | Natural | Injectable hydrogel | hDPCSs cells | Induced dentin regeneration for pulp tissue | [96] | |
| Chrysin (a kind of plant flavonoid) | Natural | Scaffold | hDPCSs cells | Increased cell viability, reduced level of TNFα, induced anti-oxidant and anti-inflammatory effects, developed mineralization in the dentin-pulp complex | [97] | |
| Vitamin E alpha-tocopherol (α-T) | Natural | Local application | Odontoblast-like MDPC-23 cells | Decreased ROS products | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedi, N.; Sajadi-Javan, Z.S.; Kouhi, M.; Ansari, L.; Khademi, A.; Ramakrishna, S. Antioxidant Materials in Oral and Maxillofacial Tissue Regeneration: A Narrative Review of the Literature. Antioxidants 2023, 12, 594. https://doi.org/10.3390/antiox12030594
Abedi N, Sajadi-Javan ZS, Kouhi M, Ansari L, Khademi A, Ramakrishna S. Antioxidant Materials in Oral and Maxillofacial Tissue Regeneration: A Narrative Review of the Literature. Antioxidants. 2023; 12(3):594. https://doi.org/10.3390/antiox12030594
Chicago/Turabian StyleAbedi, Niloufar, Zahra Sadat Sajadi-Javan, Monireh Kouhi, Legha Ansari, Abbasali Khademi, and Seeram Ramakrishna. 2023. "Antioxidant Materials in Oral and Maxillofacial Tissue Regeneration: A Narrative Review of the Literature" Antioxidants 12, no. 3: 594. https://doi.org/10.3390/antiox12030594