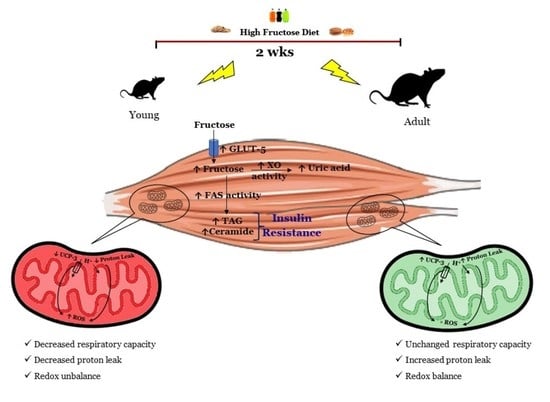
Age-Dependent Skeletal Muscle Mitochondrial Response to Short-Term Increased Dietary Fructose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Glucose Tolerance Test
2.3. Skeletal Muscle Composition
2.4. Fatty Acid Synthase Activity
2.5. Preparation of Skeletal Muscle Isolated Mitochondria
2.6. Oxidative Stress Parameters
2.7. Mitochondrial Oxidative Capacities, Marker Enzymes and Proton Leak Kinetics in Skeletal Muscles
2.8. Western Blotting of Skeletal Muscle Proteins
2.9. Statistical Analysis
2.10. Materials
3. Results
3.1. Skeletal Muscle Insulin Signaling
3.2. Skeletal Muscle Composition
3.3. Skeletal Muscle Oxidative Status
3.4. Mitochondrial Enzymes and Oxygen Consumption
3.5. Mitochondrial Proton Leak
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543, Erratum in Am. J. Clin. Nutr. 2004, 80, 1090. [Google Scholar] [CrossRef] [Green Version]
- Stricker, S.; Rudloff, S.; Geier, A.; Steveling, A.; Roeb, E.; Zimmer, K.P. Fructose Consumption-Free Sugars and Their Health Effects. Dtsch. Arztebl. Int. 2021, 118, 71–78. [Google Scholar] [CrossRef]
- Bray, G.A. Soft drink consumption and obesity: It is all about fructose. Curr. Opin. Lipidol. 2010, 21, 51–57. [Google Scholar] [CrossRef]
- Magriplis, E.; Michas, G.; Petridi, E.; Chrousos, G.P.; Roma, E.; Benetou, V.; Cholopoulos, N.; Micha, R.; Panagiotakos, D.; Zampelas, A. Dietary Sugar Intake and Its Association with Obesity in Children and Adolescents. Children 2021, 8, 676. [Google Scholar] [CrossRef]
- Baena, M.; Sangüesa, G.; Dávalos, A.; Latasa, M.J.; Sala-Vila, A.; Sánchez, R.M.; Roglans, N.; Laguna, J.C.; Alegret, M. Fructose, but not glucose, impairs insulin signaling in the three major insulin-sensitive tissues. Sci. Rep. 2016, 6, 26149. [Google Scholar] [CrossRef] [Green Version]
- Lê, K.A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Boesch, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, M.K.; Turner, N. Mitochondrial dysfunction and insulin resistance: An update. Endocr. Connect. 2015, 4, R1–R15. [Google Scholar] [CrossRef] [Green Version]
- Evans, P.L.; McMillin, S.L.; Weyrauch, L.A.; Witczak, C.A. Regulation of Skeletal Muscle Glucose Transport and Glucose Metabolism by Exercise Training. Nutrients 2019, 11, 2432. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, N.; Maurya, C.K.; Arha, D.; Avisetti, D.R.; Prathapan, A.; Raj, P.S.; Raghu, K.G.; Kalivendi, S.V.; Tamrakar, A.K. Fructose induces mitochondrial dysfunction and triggers apoptosis in skeletal muscle cells by provoking oxidative stress. Apoptosis 2015, 20, 930–947. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef] [Green Version]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Garcia, R.; Shulman, G.I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 2004, 350, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Han, D.H.; Hancock, C.R.; Jung, S.R.; Higashida, K.; Kim, S.H.; Holloszy, J.O. Deficiency of the mitochondrial electron transport chain in muscle does not cause insulin resistance. PLoS ONE. 2011, 6, e19739. [Google Scholar] [CrossRef] [Green Version]
- Boushel, R.; Gnaiger, E.; Schjerling, P.; Skovbro, M.; Kraunsøe, R.; Dela, F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 2007, 50, 790–796. [Google Scholar] [CrossRef] [Green Version]
- Crescenzo, R.; Cigliano, L.; Mazzoli, A.; Cancelliere, R.; Carotenuto, R.; Tussellino, M.; Liverini, G.; Iossa, S. Early Effects of a Low Fat, Fructose-Rich Diet on Liver Metabolism, Insulin Signaling, and Oxidative Stress in Young and Adult Rats. Front. Physiol. 2018, 9, 411. [Google Scholar] [CrossRef] [Green Version]
- Cigliano, L.; Spagnuolo, M.S.; Crescenzo, R.; Cancelliere, R.; Iannotta, L.; Mazzoli, A.; Liverini, G.; Iossa, S. Short-Term Fructose Feeding Induces Inflammation and Oxidative Stress in the Hippocampus of Young and Adult Rats. Mol. Neurobiol. 2018, 55, 2869–2883. [Google Scholar] [CrossRef]
- Benedé-Ubieto, R.; Estévez-Vázquez, O.; Ramadori, P.; Cubero, F.J.; Nevzorova, Y.A. Guidelines and Considerations for Metabolic Tolerance Tests in Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 439–450. [Google Scholar] [CrossRef] [Green Version]
- Bowe, J.E.; Franklin, Z.J.; Hauge-Evans, A.C.; King, A.J.; Persaud, S.J.; Jones, P.M. Metabolic phenotyping guidelines: Assessing glucose homeostasis in rodent models. J. Endocrinol. 2014, 222, G13–G25. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Matsuda, M.; Balas, B.; DeFronzo, R.A. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diab Care 2007, 30, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Roehrig, K.L.; Allred, J.B. Direct enzymatic procedure for the determination of liver glycogen. Anal. Biochem. 1974, 58, 414–421. [Google Scholar] [CrossRef]
- Crescenzo, R.; Bianco, F.; Coppola, P.; Mazzoli, A.; Valiante, S.; Liverini, G.; Iossa, S. Adipose tissue remodeling in rats exhibiting fructose-induced obesity. Eur. J. Nutr. 2014, 53, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, M.A.S.; Custódio, J.B.A.; Santos, M.S.; Moreno, A.J.M.; Vicente, J.A.F. Tetrandrine concentrations not affecting oxidative phosphorylation protect rat liver mitochondria from oxidative stress. Mitochondrion 2006, 6, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Flohé, L.; Otting, F. Superoxide dismutase assays. Meth. Enzymol. 1984, 105, 93–104. [Google Scholar]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Meth. Biochem. Anal. 1954, 1, 357–424. [Google Scholar]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Bettaieb, A.; Vazquez Prieto, M.A.; Rodriguez Lanzi, C.; Miatello, R.M.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. 5(-)-Epicatechin mitigates high-fructose-associated insulin resistance by modulating redox signaling and endoplasmic reticulum stress. Free Radic. Biol. Med. 2014, 72, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Srere, P.A. Citrate synthase. Meth. Enzymol. 1969, 13, 3–11. [Google Scholar]
- Lionetti, L.; Mollica, M.P.; Crescenzo, R.; D’Andrea, E.; Ferraro, M.; Bianco, F.; Liverini, G.; Iossa, S. Skeletal muscle subsarcolemmal mitochondrial dysfunction in high-fat fed rats exhibiting impaired glucose homeostasis. Int. J. Obes (Lond) 2007, 31, 1596–1604. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, J.; Di, L.J. Glycogen synthesis and beyond, a comprehensive review of GSK3 as a key regulator of metabolic pathways and a therapeutic target for treating metabolic diseases. Med. Res. Rev. 2022, 42, 946–982. [Google Scholar] [CrossRef]
- Coen, P.M.; Goodpaster, B.H. Role of intramyocelluar lipids in human health. Trends Endocrinol. Metab. 2012, 23, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Jani, S.; Da Eira, D.; Hadday, I.; Bikopoulos, G.; Mohasses, A.; de Pinho, R.A.; Ceddia, R.B. Distinct mechanisms involving diacylglycerol, ceramides, and inflammation underlie insulin resistance in oxidative and glycolytic muscles from high fat-fed rats. Sci. Rep. 2021, 11, 19160. [Google Scholar] [CrossRef]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef] [Green Version]
- Sokolowska, E.; Blachnio-Zabielska, A. The Role of Ceramides in Insulin Resistance. Front. Endocrinol. (Lausanne) 2019, 10, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Busserolles, J.; Rock, E.; Gueux, E.; Mazur, A.; Grolier, P.; Rayssiguier, Y. Short-term consumption of a high-sucrose diet has a pro-oxidant effect in rats. Br. J. Nutr. 2002, 87, 337–342. [Google Scholar] [CrossRef]
- Girard, A.; Madani, S.; Boukortt, F.; Cherkaoui-Malki, M.; Belleville, J.; Prost, J. Fructose-enriched diet modifies antioxidant status and lipid metabolism in spontaneously hypertensive rats. Nutrition 2006, 22, 758–766. [Google Scholar] [CrossRef]
- Yen, T.C.; King, K.L.; Lee, H.C.; Yeh, S.H.; Wei, Y.H. Age-dependent increase of mitochondrial DNA deletions together with lipid peroxides and superoxide dismutase in human liver mitochondria. Free Radic. Biol. Med. 1994, 16, 207–214. [Google Scholar] [CrossRef]
- Yang, W.; Burkhardt, B.; Fischer, L.; Beirow, M.; Bork, N.; Wönne, E.C.; Wagner, C.; Husen, B.; Zeilinger, K.; Liu, L.; et al. Age-dependent changes of the antioxidant system in rat livers are accompanied by altered MAPK activation and a decline in motor signaling. EXCLI J. 2015, 14, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, R.; Bianco, F.; Mazzoli, A.; Giacco, A.; Liverini, G.; Iossa, S. Skeletal muscle mitochondrial energetic efficiency and aging. Int. J. Mol. Sci. 2015, 16, 10674–10685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangwung, P.; Petersen, K.F.; Shulman, G.I.; Knowles, J.W. Mitochondrial Dysfunction, Insulin Resistance, and Potential Genetic Implications. Endocrinology 2020, 161, bqaa017. [Google Scholar] [CrossRef]
- Brand, M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010, 45, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Muller, F.L.; Liu, Y.; Van Remmen, H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004, 279, 49064–49073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heather, L.C.; Carr, C.A.; Stuckey, D.J.; Pope, S.; Morten, K.J.; Carter, E.E.; Edwards, L.M.; Clarke, K. Critical role of complex III in the early metabolic changes following myocardial infarction. Cardiovasc. Res. 2010, 85, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Harper, M.E.; Green, K.; Brand, M.D. The efficiency of cellular energy transduction and its implications for obesity. Ann. Rev. Nutr. 2008, 28, 13–33. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Brand, M.D. The regulation and physiology of mitochondrial proton leak. Physiology (Bethesda) 2011, 26, 192–205. [Google Scholar] [CrossRef]
- Codella, R.; Alves, T.C.; Befroy, D.E.; Choi, C.S.; Luzi, L.; Rothman, D.L.; Kibbey, R.G.; Shulman, G.I. Overexpression of UCP3 decreases mitochondrial efficiency in mouse skeletal muscle in vivo. FEBS Lett. 2022. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, G.; Bo, H.; Qu, J.; Ma, G.; Cao, D.; Wen, L.; Liu, S.; Ji, L.L.; Zhang, Y. Upregulation of uncoupling protein-3 in skeletal muscle during exercise: A potential antioxidant function. Free Radic. Biol. Med. 2009, 46, 138–145. [Google Scholar] [CrossRef]
- Barreiro, E.; Garcia-Martínez, C.; Mas, S.; Ametller, E.; Gea, J.; Argilés, J.M.; Busquets, S.; López-Soriano, F.J. UCP3 overexpression neutralizes oxidative stress rather than nitrosative stress in mouse myotubes. FEBS Lett. 2009, 583, 350–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toime, L.J.; Brand, M.D. Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radic Biol. Med. 2010, 49, 606–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crescenzo, R.; Bianco, F.; Coppola, P.; Mazzoli, A.; Cigliano, L.; Liverini, G.; Iossa, S. Increased skeletal muscle mitochondrial efficiency in rats with fructose-induced alteration in glucose tolerance. Br. J. Nutr. 2013, 110, 1996–2003. [Google Scholar] [CrossRef] [PubMed]






| Component, g/100 g | Control Diet | Fructose Diet |
|---|---|---|
| Standard Chow a | 50.5 | 50.5 |
| Sunflower Oil | 1.5 | 1.5 |
| Casein | 9.2 | 9.2 |
| Alphacel | 9.8 | 9.8 |
| Cornstarch | 20.4 | --- |
| Fructose | --- | 20.4 |
| Water | 6.4 | 6.4 |
| AIN-76 mineral mix | 1.6 | 1.6 |
| AIN-76 vitamin mix | 0.4 | 0.4 |
| Choline | 0.1 | 0.1 |
| Methionine | 0.1 | 0.1 |
| Energy content and composition | ||
| Gross Energy Density (kJ/g) | 17.2 | 17.2 |
| ME content (kJ/g) b | 11.1 | 11.1 |
| Proteins (% ME) | 29.0 | 29.0 |
| Lipids (% ME) | 10.6 | 10.6 |
| Carbohydrates (% ME) | 60.4 | 60.4 |
| Of which: | ||
| Fructose | --- | 30.0 |
| Starch | 52.8 | 22.8 |
| Sugars | 7.6 | 7.6 |
| Parameter | Diet Effect | Age Effect | |
|---|---|---|---|
| Young | Adult | ||
| Insulin signaling | |||
| Skeletal muscle insulin sensitivity index | ↓ | ↓ | ns |
| Insulin signalling (pAkt, p-GSK, GLUT-4) | ↓ | ↓ | ns |
| Glycogen | ↓ | ↓ | ↑ |
| Composition | |||
| Fructose, Uric Acid, GLUT-5 | ↑ | ↑ | ns |
| Triglycerides and ceramide | ↑ | ↑ | ns |
| Fatty Acid Synthase | ↑ | ↑ | ns |
| Redox homeostasis | |||
| TBARS | ↑ | ns | ↑ |
| Mitochondrial TBARS | ↑ | ns | ↑ |
| NADPH oxidase | ↑ | ↑ | ns |
| xanthine oxidase | ↑ | ↑ | ↑ |
| Catalase | ↓ | ns | ns |
| Superoxide dismutase | ↓ | ns | ns |
| Mitochondrial superoxide dismutase | ↓ | ↓ | ↑ |
| Mitochondrial function | |||
| Activity of COX and CS | ↓ | ns | ↓ |
| State 3 respiration | ↓ | ns | ns |
| Complex III amount | ↓ | ns | ns |
| Basal proton leak | ↓ | ↑ | ↓ |
| Palmitate-induced proton leak | ↓ | ↑ | ↓ |
| UCP-3 | ↓ | ↑ | ↓ |
| ANT | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatto, C.; Di Porzio, A.; Crescenzo, R.; Barrella, V.; Iossa, S.; Mazzoli, A. Age-Dependent Skeletal Muscle Mitochondrial Response to Short-Term Increased Dietary Fructose. Antioxidants 2023, 12, 299. https://doi.org/10.3390/antiox12020299
Gatto C, Di Porzio A, Crescenzo R, Barrella V, Iossa S, Mazzoli A. Age-Dependent Skeletal Muscle Mitochondrial Response to Short-Term Increased Dietary Fructose. Antioxidants. 2023; 12(2):299. https://doi.org/10.3390/antiox12020299
Chicago/Turabian StyleGatto, Cristina, Angela Di Porzio, Raffaella Crescenzo, Valentina Barrella, Susanna Iossa, and Arianna Mazzoli. 2023. "Age-Dependent Skeletal Muscle Mitochondrial Response to Short-Term Increased Dietary Fructose" Antioxidants 12, no. 2: 299. https://doi.org/10.3390/antiox12020299