Extracellular Vesicles and Their Renin–Angiotensin Cargo as a Link between Metabolic Syndrome and Parkinson’s Disease
Abstract
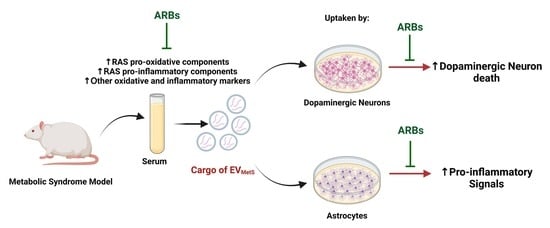
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Isolation of EVs from Animal Serum
2.3. EV Characterization by Western Blot, Transmission Electron Microscopy (TEM), and Esterase Activity
2.4. EV-RAS Protein Analysis by Exoview®
2.5. EV-mRNA Cargo Analysis
2.6. Cultures of the N27 Dopaminergic Cell Line and the C6 Astrocytic Cell Line
2.7. EV Labeling and Uptaking by the N27 Dopaminergic Cell Line and the C6 Astrocytic Cell Line
2.8. Culture Treatments and MTT Assay
2.9. NADPH-Oxidase Activity and Cytokine Levels
2.10. Analysis of Cell mRNA Expression in Recipient Cells
2.11. Statistical Analysis
3. Results
3.1. Isolation and Characterization of EVs from Rat Serum and Effects of MetS and Candesartan Treatment
3.2. mRNA Expression of RAS Components in Serum EVs and Effects of Treatment of MetS Rats with Candesartan
3.3. RAS Component Proteins in EVs and Effects of MetS and Treatment with Candesartan
3.4. mRNA Expression of Pro-Inflammatory Cytokines and Pro-Oxidative Markers in Serum EVs and Effects of Treatment of MetS Rats with Candesartan
3.5. Uptake of EVMetS by the N27 Dopaminergic Cell Line and the C6 Astrocytic Cell Line In Vitro
3.6. EVMetS Enhance N27 Dopaminergic Cell Line Death in Cell Cultures Mediated by AT1 Receptor Activation
3.7. EVMetS Modulate the C6 Astrocytic Cell Line and N27 Dopaminergic Cell Line Function through AT1 Receptor Activation
3.8. EVMetS Uptake Leads to RAS Dysregulation in Cultured N27 Dopaminergic Cell Line and C6 Astrocytic Cell Line
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakrabarti, S.; Bisaglia, M. Oxidative Stress and Neuroinflammation in Parkinson’s Disease: The Role of Dopamine Oxidation Products. Antioxidants 2023, 12, 955. [Google Scholar] [CrossRef]
- Hassanzadeh, K.; Rahimmi, A. Oxidative stress and neuroinflammation in the story of Parkinson’s disease: Could targeting these pathways write a good ending? J. Cell Physiol. 2018, 234, 23–32. [Google Scholar] [CrossRef]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef]
- Kusters, C.D.J.; Paul, K.C.; Duarte Folle, A.; Keener, A.M.; Bronstein, J.M.; Bertram, L.; Hansen, J.; Horvath, S.; Sinsheimer, J.S.; Lill, C.M.; et al. Increased Menopausal Age Reduces the Risk of Parkinson’s Disease: A Mendelian Randomization Approach. Mov. Disord. 2021, 36, 2264–2272. [Google Scholar] [CrossRef]
- Rocca, W.A.; Smith, C.Y.; Gazzuola Rocca, L.; Savica, R.; Mielke, M.M. Association of Premenopausal Bilateral Oophorectomy With Parkinsonism and Parkinson Disease. JAMA Netw. Open 2022, 5, e2238663. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.E.; Kim, S.M.; Han, K.; Kim, N.H.; Chung, H.S.; Kim, J.W.; Han, B.; Cho, S.J.; Yu, J.H.; Park, Y.G.; et al. Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLoS Med. 2018, 15, e1002640. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.A.; Labandeira, C.M.; Valenzuela, R.; Quijano, A.; Sanchez-Andrade, M.; Suarez-Quintanilla, J.A.; Lanciego, J.L.; Labandeira-Garcia, J.L.; Rodriguez-Perez, A.I. AT1 receptor autoantibodies mediate effects of metabolic syndrome on dopaminergic vulnerability. Brain Behav. Immun. 2023, 108, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Dong, S.; Tao, Y.; Huo, Y.; Zhou, Z.; Huang, W.; Qu, H.; Liu, J.; Chen, Y.; Xu, Z.; et al. Metabolic syndrome contributes to cognitive impairment in patients with Parkinson’s disease. Park. Relat. Disord. 2018, 55, 68–74. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabres, M.; Capo, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Moustaid-Moussa, N. The renin-angiotensin system: A link between obesity, inflammation and insulin resistance. Obes. Rev. 2012, 13, 136–149. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, A.D.; Krause, E.G.; Woods, S.C. The renin angiotensin system and the metabolic syndrome. Physiol. Behav. 2010, 100, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Kamath, T.; Abdulraouf, A.; Burris, S.J.; Langlieb, J.; Gazestani, V.; Nadaf, N.M.; Balderrama, K.; Vanderburg, C.; Macosko, E.Z. Single-cell genomic profiling of human dopamine neurons identifies a population that selectively degenerates in Parkinson’s disease. Nat. Neurosci. 2022, 25, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Valenzuela, R.; Borrajo, A.; Rodriguez-Perez, A.I. Brain renin-angiotensin system and dopaminergic cell vulnerability. Front. Neuroanat. 2014, 8, 67. [Google Scholar] [CrossRef]
- Rodriguez-Perez, A.I.; Sucunza, D.; Pedrosa, M.A.; Garrido-Gil, P.; Kulisevsky, J.; Lanciego, J.L.; Labandeira-Garcia, J.L. Angiotensin Type 1 Receptor Antagonists Protect Against Alpha-Synuclein-Induced Neuroinflammation and Dopaminergic Neuron Death. Neurotherapeutics 2018, 15, 1063–1081. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Valenzuela, R.; Costa-Besada, M.A.; Villar-Cheda, B.; Rodriguez-Perez, A.I. The intracellular renin-angiotensin system: Friend or foe. Some light from the dopaminergic neurons. Prog. Neurobiol. 2021, 199, 101919. [Google Scholar] [CrossRef]
- Saravi, B.; Li, Z.; Lang, C.N.; Schmid, B.; Lang, F.K.; Grad, S.; Alini, M.; Richards, R.G.; Schmal, H.; Sudkamp, N.; et al. The Tissue Renin-Angiotensin System and Its Role in the Pathogenesis of Major Human Diseases: Quo Vadis? Cells 2021, 10, 650. [Google Scholar] [CrossRef]
- Jackson, L.; Eldahshan, W.; Fagan, S.C.; Ergul, A. Within the Brain: The Renin Angiotensin System. Int. J. Mol. Sci. 2018, 19, 876. [Google Scholar] [CrossRef]
- Dominguez-Meijide, A.; Rodriguez-Perez, A.I.; Diaz-Ruiz, C.; Guerra, M.J.; Labandeira-Garcia, J.L. Dopamine modulates astroglial and microglial activity via glial renin-angiotensin system in cultures. Brain Behav. Immun. 2017, 62, 277–290. [Google Scholar] [CrossRef]
- Milsted, A.; Barna, B.P.; Ransohoff, R.M.; Brosnihan, K.B.; Ferrario, C.M. Astrocyte cultures derived from human brain tissue express angiotensinogen mRNA. Proc. Natl. Acad. Sci. USA 1990, 87, 5720–5723. [Google Scholar] [CrossRef] [PubMed]
- Stornetta, R.L.; Hawelu-Johnson, C.L.; Guyenet, P.G.; Lynch, K.R. Astrocytes synthesize angiotensinogen in brain. Science 1988, 242, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Booth, H.D.E.; Hirst, W.D.; Wade-Martins, R. The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, B.; Kodali, M.C.; Chen, C.; Kim, E.; Patters, B.J.; Lan, L.; Kumar, S.; Wang, X.; Yue, J.; et al. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J. Neuroinflamm. 2018, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Morales-Prieto, D.M.; Murrieta-Coxca, J.M.; Stojiljkovic, M.; Diezel, C.; Streicher, P.E.; Henao-Restrepo, J.A.; Rostel, F.; Lindner, J.; Witte, O.W.; Weis, S.; et al. Small Extracellular Vesicles from Peripheral Blood of Aged Mice Pass the Blood-Brain Barrier and Induce Glial Cell Activation. Cells 2022, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular Vesicles under Oxidative Stress Conditions: Biological Properties and Physiological Roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Zheng, J.C. Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases. Transl. Neurodegener. 2022, 11, 53. [Google Scholar] [CrossRef]
- Akbar, N.; Azzimato, V.; Choudhury, R.P.; Aouadi, M. Extracellular vesicles in metabolic disease. Diabetologia 2019, 62, 2179–2187. [Google Scholar] [CrossRef]
- Eguchi, A.; Lazic, M.; Armando, A.M.; Phillips, S.A.; Katebian, R.; Maraka, S.; Quehenberger, O.; Sears, D.D.; Feldstein, A.E. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J. Mol. Med. 2016, 94, 1241–1253. [Google Scholar] [CrossRef]
- Lee, J.E.; Moon, P.G.; Lee, I.K.; Baek, M.C. Proteomic Analysis of Extracellular Vesicles Released by Adipocytes of Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Protein J. 2015, 34, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Novaes, A.; Borges, F.T.; Maquigussa, E.; Varela, V.A.; Dias, M.V.S.; Boim, M.A. Influence of high glucose on mesangial cell-derived exosome composition, secretion and cell communication. Sci. Rep. 2019, 9, 6270. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Ye, C.; Ren, X.S.; Qiu, Y.; Zang, Y.H.; Xiong, X.Q.; Wang, J.J.; Chen, Q.; Li, Y.H.; Kang, Y.M.; et al. Exosome-Mediated Transfer of ACE (Angiotensin-Converting Enzyme) From Adventitial Fibroblasts of Spontaneously Hypertensive Rats Promotes Vascular Smooth Muscle Cell Migration. Hypertension 2018, 72, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Wang, H.; Li, B.; Qin, Q.; Qi, L.; Nagarkatti, M.; Nagarkatti, P.; Janicki, J.S.; Wang, X.L.; Cui, T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 89, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Ramos-Zaldivar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalan, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood-brain barrier: A review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef]
- Pettersson, U.S.; Walden, T.B.; Carlsson, P.O.; Jansson, L.; Phillipson, M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef]
- Khan, N.Z.; Cao, T.; He, J.; Ritzel, R.M.; Li, Y.; Henry, R.J.; Colson, C.; Stoica, B.A.; Faden, A.I.; Wu, J. Spinal cord injury alters microRNA and CD81+ exosome levels in plasma extracellular nanoparticles with neuroinflammatory potential. Brain Behav. Immun. 2021, 92, 165–183. [Google Scholar] [CrossRef]
- Arab, T.; Mallick, E.R.; Huang, Y.; Dong, L.; Liao, Z.; Zhao, Z.; Gololobova, O.; Smith, B.; Haughey, N.J.; Pienta, K.J.; et al. Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single-particle analysis platforms. J. Extracell. Vesicles 2021, 10, e12079. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Conlon, M.M.; Rider, M.A.; Brownstein, N.C.; Meckes, D.G., Jr. Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. J. Extracell. Vesicles 2016, 5, 31295. [Google Scholar] [CrossRef]
- Daaboul, G.G.; Gagni, P.; Benussi, L.; Bettotti, P.; Ciani, M.; Cretich, M.; Freedman, D.S.; Ghidoni, R.; Ozkumur, A.Y.; Piotto, C.; et al. Digital Detection of Exosomes by Interferometric Imaging. Sci. Rep. 2016, 6, 37246. [Google Scholar] [CrossRef]
- Camino, T.; Lago-Baameiro, N.; Bravo, S.B.; Molares-Vila, A.; Sueiro, A.; Couto, I.; Baltar, J.; Casanueva, E.F.; Pardo, M. Human obese white adipose tissue sheds depot-specific extracellular vesicles and reveals candidate biomarkers for monitoring obesity and its comorbidities. Transl. Res. 2022, 239, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Camino, T.; Lago-Baameiro, N.; Pardo, M. Extracellular Vesicles as Carriers of Adipokines and Their Role in Obesity. Biomedicines 2023, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Barroso-Chinea, P.; Villar-Cheda, B.; Joglar, B.; Munoz, A.; Lanciego, J.L.; Labandeira-Garcia, J.L. Location of prorenin receptors in primate substantia nigra: Effects on dopaminergic cell death. J. Neuropathol. Exp. Neurol. 2010, 69, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Rodriguez-Perez, A.I.; Costa-Besada, M.A.; Rivas-Santisteban, R.; Garrido-Gil, P.; Lopez-Lopez, A.; Navarro, G.; Lanciego, J.L.; Franco, R.; Labandeira-Garcia, J.L. An ACE2/Mas-related receptor MrgE axis in dopaminergic neuron mitochondria. Redox. Biol. 2021, 46, 102078. [Google Scholar] [CrossRef] [PubMed]
- Labandeira, C.M.; Pedrosa, M.A.; Quijano, A.; Valenzuela, R.; Garrido-Gil, P.; Sanchez-Andrade, M.; Suarez-Quintanilla, J.A.; Rodriguez-Perez, A.I.; Labandeira-Garcia, J.L. Angiotensin type-1 receptor and ACE2 autoantibodies in Parkinson s disease. NPJ Park. Dis. 2022, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, A.; Valenzuela, R.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L.; Munoz, A. Interactions between Angiotensin Type-1 Antagonists, Statins, and ROCK Inhibitors in a Rat Model of L-DOPA-Induced Dyskinesia. Antioxidants 2023, 12, 1454. [Google Scholar] [CrossRef]
- Parga, J.A.; Rodriguez-Perez, A.I.; Garcia-Garrote, M.; Rodriguez-Pallares, J.; Labandeira-Garcia, J.L. Angiotensin II induces oxidative stress and upregulates neuroprotective signaling from the NRF2 and KLF9 pathway in dopaminergic cells. Free Radic. Biol. Med. 2018, 129, 394–406. [Google Scholar] [CrossRef]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef]
- Hong, H.; Zeng, J.S.; Kreulen, D.L.; Kaufman, D.I.; Chen, A.F. Atorvastatin protects against cerebral infarction via inhibition of NADPH oxidase-derived superoxide in ischemic stroke. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2210–H2215. [Google Scholar] [CrossRef]
- Gupta, S.; Knowlton, A.A. HSP60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3052–H3056. [Google Scholar] [CrossRef]
- Savina, A.; Vidal, M.; Colombo, M.I. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 2002, 115, 2505–2515. [Google Scholar] [CrossRef]
- Ferrante, S.C.; Nadler, E.P.; Pillai, D.K.; Hubal, M.J.; Wang, Z.; Wang, J.M.; Gordish-Dressman, H.; Koeck, E.; Sevilla, S.; Wiles, A.A.; et al. Adipocyte-derived exosomal miRNAs: A novel mechanism for obesity-related disease. Pediatr. Res. 2015, 77, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.S.; Premaratna, S.D.; Bennett, G.; Lambrou, M.; Stahl, L.A.; Jois, M.; Barber, E.; Antoniadis, C.P.; Woods, S.C.; Cameron-Smith, D.; et al. Inhibition of the Renin-Angiotensin System Reduces Gene Expression of Inflammatory Mediators in Adipose Tissue Independent of Energy Balance. Front. Endocrinol. 2021, 12, 682726. [Google Scholar] [CrossRef] [PubMed]
- Frigolet, M.E.; Torres, N.; Tovar, A.R. The renin-angiotensin system in adipose tissue and its metabolic consequences during obesity. J. Nutr. Biochem. 2013, 24, 2003–2015. [Google Scholar] [CrossRef]
- Kintscher, U.; Bramlage, P.; Paar, W.D.; Thoenes, M.; Unger, T. Irbesartan for the treatment of hypertension in patients with the metabolic syndrome: A sub analysis of the Treat to Target post authorization survey. Prospective observational, two armed study in 14,200 patients. Cardiovasc. Diabetol. 2007, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Kim, S.; Ye, B.S.; Lee, E.; Yu, Y.M. Protective Effect of Renin-Angiotensin System Inhibitors on Parkinson’s Disease: A Nationwide Cohort Study. Front. Pharmacol. 2022, 13, 837890. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Tseng, Y.F.; Shen, A.L.; Chao, J.C.; Hsu, C.Y.; Lin, H.L. Association of Angiotensin Receptor Blockers with Incident Parkinson Disease in Patients with Hypertension: A Retrospective Cohort Study. Am. J. Med. 2022, 135, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.Y.; Leng, Y.; Wang, Z.X.; Xiao, X.; Zhang, X.; Wen, T.; Gong, H.Z.; Hong, A.; Ma, Y. Exosomal transfer of obesity adipose tissue for decreased miR-141-3p mediate insulin resistance of hepatocytes. Int. J. Biol. Sci. 2019, 15, 351–368. [Google Scholar] [CrossRef]
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; LeGonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S.; et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res. 2016, 76, 4051–4057. [Google Scholar] [CrossRef]
- Hara, Y.; Wakino, S.; Tanabe, Y.; Saito, M.; Tokuyama, H.; Washida, N.; Tatematsu, S.; Yoshioka, K.; Homma, K.; Hasegawa, K.; et al. Rho and Rho-kinase activity in adipocytes contributes to a vicious cycle in obesity that may involve mechanical stretch. Sci. Signal. 2011, 4, ra3. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ma, L.; Zhang, W.; Yang, W.; Feng, Q.; Wang, H. Extracellular signals regulate the biogenesis of extracellular vesicles. Biol. Res. 2022, 55, 35. [Google Scholar] [CrossRef]
- Silverman, J.M.; Reiner, N.E. Exosomes and other microvesicles in infection biology: Organelles with unanticipated phenotypes. Cell Microbiol. 2011, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liang, X.; Dai, F.; Guan, H.; Sun, J.; Yao, W. RhoA/ROCK Pathway Activation is Regulated by AT1 Receptor and Participates in Smooth Muscle Migration and Dedifferentiation via Promoting Actin Cytoskeleton Polymerization. Int. J. Mol. Sci. 2020, 21, 5398. [Google Scholar] [CrossRef]
- Rodriguez-Perez, A.I.; Borrajo, A.; Rodriguez-Pallares, J.; Guerra, M.J.; Labandeira-Garcia, J.L. Interaction between NADPH-oxidase and Rho-kinase in angiotensin II-induced microglial activation. Glia 2015, 63, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Ravarotto, V.; Pagnin, E.; Maiolino, G.; Fragasso, A.; Carraro, G.; Rossi, B.; Calo, L.A. The blocking of angiotensin II type 1 receptor and RhoA/Rho kinase activity in hypertensive patients: Effect of olmesartan medoxomil and implication with cardiovascular-renal remodeling. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 1245–1250. [Google Scholar] [CrossRef]
- Yang, Y.; Boza-Serrano, A.; Dunning, C.J.R.; Clausen, B.H.; Lambertsen, K.L.; Deierborg, T. Inflammation leads to distinct populations of extracellular vesicles from microglia. J. Neuroinflamm. 2018, 15, 168. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Bernuz, M.; Puerta, R.; Esteban de Antonio, E.; Sanchez-Lopez, E.; Souto, E.B.; Camins, A.; Marti, M.; Pividori, M.I.; et al. Extracellular vesicles, the emerging mirrors of brain physiopathology. Int. J. Biol. Sci. 2023, 19, 721–743. [Google Scholar] [CrossRef]
- Gupte, M.; Boustany-Kari, C.M.; Bharadwaj, K.; Police, S.; Thatcher, S.; Gong, M.C.; English, V.L.; Cassis, L.A. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R781–R788. [Google Scholar] [CrossRef]
- Pinheiro, T.A.; Barcala-Jorge, A.S.; Andrade, J.M.O.; Pinheiro, T.A.; Ferreira, E.C.N.; Crespo, T.S.; Batista-Jorge, G.C.; Vieira, C.A.; Lelis, D.F.; Paraiso, A.F.; et al. Obesity and malnutrition similarly alter the renin-angiotensin system and inflammation in mice and human adipose. J. Nutr. Biochem. 2017, 48, 74–82. [Google Scholar] [CrossRef]
- Engeli, S.; Schling, P.; Gorzelniak, K.; Boschmann, M.; Janke, J.; Ailhaud, G.; Teboul, M.; Massiera, F.; Sharma, A.M. The adipose-tissue renin-angiotensin-aldosterone system: Role in the metabolic syndrome? Int. J. Biochem. Cell Biol. 2003, 35, 807–825. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.H.; Standridge, M.K.; Taylor, J.W.; Moustaid, N. Angiotensinogen gene expression in adipose tissue: Analysis of obese models and hormonal and nutritional control. Am. J. Physiol. 1997, 273, R236–R242. [Google Scholar] [CrossRef]
- Vargas-Castillo, A.; Tobon-Cornejo, S.; Del Valle-Mondragon, L.; Torre-Villalvazo, I.; Schcolnik-Cabrera, A.; Guevara-Cruz, M.; Pichardo-Ontiveros, E.; Fuentes-Romero, R.; Bader, M.; Alenina, N.; et al. Angiotensin-(1-7) induces beige fat thermogenesis through the Mas receptor. Metabolism 2020, 103, 154048. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Tamura, K.; Wakui, H.; Ohsawa, M.; Azushima, K.; Uneda, K.; Kanaoka, T.; Kobayashi, R.; Ohki, K.; Matsuda, M.; et al. Effects of the Angiotensin receptor blocker olmesartan on adipocyte hypertrophy and function in mice with metabolic disorders. BioMed Res. Int. 2014, 2014, 946492. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Haron, S.; Kilmister, E.J.; Davis, P.F.; Stylli, S.S.; Mantamadiotis, T.; Kaye, A.H.; Hall, S.R.; Tan, S.T.; Wickremesekera, A.C. The renin-angiotensin system in central nervous system tumors and degenerative diseases. Front. Biosci. (Landmark Ed.) 2021, 26, 628–642. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Zhang, Z.; Zhang, X.; Zhu, Y.; Zhang, C.; Bi, Y. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab. 2022, 34, 1264–1279.e8. [Google Scholar] [CrossRef]
- Bhat, S.A.; Goel, R.; Shukla, S.; Shukla, R.; Hanif, K. Angiotensin Receptor Blockade by Inhibiting Glial Activation Promotes Hippocampal Neurogenesis Via Activation of Wnt/beta-Catenin Signaling in Hypertension. Mol. Neurobiol. 2018, 55, 5282–5298. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Zhao, S.; Sheng, S.; Wang, Y.; Ding, L.; Xu, X.; Xia, X.; Zheng, J.C. Astrocyte-derived extracellular vesicles: A double-edged sword in central nervous system disorders. Neurosci. Biobehav. Rev. 2021, 125, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.D.; Dastgheyb, R.M.; Yoo, S.W.; Trout, A.; Talbot, C.C., Jr.; Hao, H.; Witwer, K.W.; Haughey, N.J. TNFalpha and IL-1beta modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 2018, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Datta Chaudhuri, A.; Dasgheyb, R.M.; DeVine, L.R.; Bi, H.; Cole, R.N.; Haughey, N.J. Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia 2020, 68, 128–144. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Borgmann, K.; Edara, V.V.; Stacy, S.; Ghorpade, A.; Ikezu, T. Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J. Extracell. Vesicles 2020, 9, 1706801. [Google Scholar] [CrossRef]
- Agouni, A.; Ducluzeau, P.H.; Benameur, T.; Faure, S.; Sladkova, M.; Duluc, L.; Leftheriotis, G.; Pechanova, O.; Delibegovic, M.; Martinez, M.C.; et al. Microparticles from patients with metabolic syndrome induce vascular hypo-reactivity via Fas/Fas-ligand pathway in mice. PLoS ONE 2011, 6, e27809. [Google Scholar] [CrossRef]








| Genes | Primers (5′-3′) | Accession Number | Product Length (bps) |
|---|---|---|---|
| Ace2 | F: GTGGAGGTGGATGGTCTTTCAGG; R: CACCAACGATCTCCCGCTTCA | NM_001012006.2 | 86 |
| Actb | F: TCGTGCGTGACATTAAAGAG; R: TGCCACAGGATTCCATACC | NM_031144.3 | 198 |
| Agt | F: GAGTGAGGCAAGAGGTGTA; R: TCCAACGATCCAAGGTAGAA | NM_134432.2 | 90 |
| Agtr1a | F: GCCAAGCCAGCCATCAGC; R: TTCAACCTCTACGCCAGTGTG; | NM_030985.4 | 149 |
| Atp6ap2 (Prr) | F: TGGTGGGAATGCAGTGGTAGAG; R: GGGACTTTGGGTGTTCTCTTGTT | NM_001007091.1 | 109 |
| Cybb (Gp91-phox) | F: ATCTTGCTGCCAGTGTGTCG; R: AATGGTGTGAATGGCCGTGTG | NM_023965.2 | 153 |
| Gapdh | F: ACATACTCAGCACCAGCAT; R: GCAAGTTCAACGGCACAGT | NM_017008.4 | 124 |
| Gfap | F: TGGAGGTGGAGAGGGACAATC; R: CTCCAGATCCACACGAGCCAA | NM_017009.2 | 152 |
| Il1b | F: GGCAACTGTCCCTGAACTCA; R: TGTCGAGATGCTGCTGTGAGA | NM_031512.2 | 170 |
| Il6 | F: TGGTATCCTCTGTGAAGTCTC; R: CAGCCAGTTGCCTTCTTG | NM_012589.2 | 91 |
| Mas1 | F: TCCCCAGACCAGTCATCCT; R: TGCTGGAGGTATTCATGGCTT | NM_012757.2 | 122 |
| Ncf1 (P47-phox) | F: TGTTCCTGGTTAAGTGGCAGGA; R: CTGGGAGCTGGGAGGTGAG | NM_053734.2 | 157 |
| Tnf | F: CACGTCGTAGCAAACCACCA; R: GGTTGTCTTTGAGATCCATGCCA | NM_012675.3 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa, M.A.; Labandeira, C.M.; Lago-Baameiro, N.; Valenzuela, R.; Pardo, M.; Labandeira-Garcia, J.L.; Rodriguez-Perez, A.I. Extracellular Vesicles and Their Renin–Angiotensin Cargo as a Link between Metabolic Syndrome and Parkinson’s Disease. Antioxidants 2023, 12, 2045. https://doi.org/10.3390/antiox12122045
Pedrosa MA, Labandeira CM, Lago-Baameiro N, Valenzuela R, Pardo M, Labandeira-Garcia JL, Rodriguez-Perez AI. Extracellular Vesicles and Their Renin–Angiotensin Cargo as a Link between Metabolic Syndrome and Parkinson’s Disease. Antioxidants. 2023; 12(12):2045. https://doi.org/10.3390/antiox12122045
Chicago/Turabian StylePedrosa, Maria A., Carmen M. Labandeira, Nerea Lago-Baameiro, Rita Valenzuela, Maria Pardo, Jose Luis Labandeira-Garcia, and Ana I. Rodriguez-Perez. 2023. "Extracellular Vesicles and Their Renin–Angiotensin Cargo as a Link between Metabolic Syndrome and Parkinson’s Disease" Antioxidants 12, no. 12: 2045. https://doi.org/10.3390/antiox12122045