Effects of In Vitro Digestion on the Antioxidant Activity of Three Phenolic Extracts from Olive Mill Wastewaters
Abstract
:1. Introduction
2. Materials and Methods
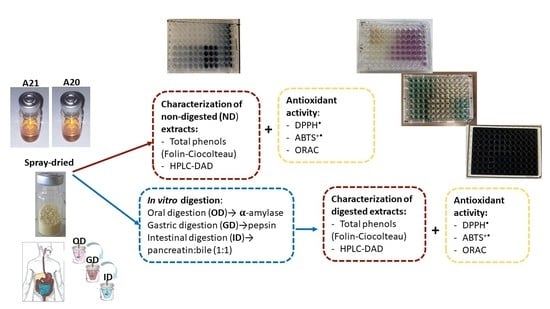
2.1. Phenolic Extract
2.2. In Vitro Digestion
2.3. Total Polyphenol (TP) Content
2.4. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH•) Inhibition
2.5. 2,2-Azinobis-(3-Ethylbenzothiazoline-6-Sulfonate) (ABTS•+) Inhibition
2.6. Oxygen Radical Absorbance Capacity (ORAC)
2.7. High-Performance Liquid Chromatography (HPLC)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Content of the Extracts before and after In Vitro Digestion
3.2. Phenolic Composition of the Extracts before and after In Vitro Digestion
3.3. Antioxidant Activity of the Extracts before and after In Vitro Digestion
3.4. Pearson’s Correlation Matrix
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iakovou, E.; Kourti, M. A comprehensive overview of the complex role of oxidative stress in aging, the contributing environmental stressors and emerging antioxidant therapeutic interventions. Front. Aging Neurosci. 2022, 14, 827900. [Google Scholar] [CrossRef] [PubMed]
- Nandita, H.; Manohar, M.; Gowda, D.V. Recent review on oxidative stress, cellular senescence and age-associated diseases. Int. J. Res. Pharm. Sci. 2020, 11, 1331–1342. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, X.; Chen, W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, A.; Khan, A.; Khan, I. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- De Ciriano, M.G.; Rehecho, S.; Calvo, M.I.; Cavero, R.Y.; Navarro, Í.; Astiasarán, I.; Ansorena, D. Effect of lyophilized water extracts of Melissa officinalis on the stability of algae and linseed oil-in-water emulsion to be used as a functional ingredient in meat products. Meat Sci. 2010, 85, 373–377. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging green techniques for the extraction of antioxidants from agri-food by-products as promising ingredients for the food industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Ramírez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, C.; Seguí, L. Valorization of vegetable fresh-processing residues as functional powder ingredients. A review on the potential impact of pretreatments and drying methods on bioactive compounds and their bioaccessibility. Front. Sustain. Food. Syst. 2021, 5, 654313. [Google Scholar] [CrossRef]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I.; Losito, I. Bioactive compounds in waste by-products from olive oil production: Applications and structural characterization by mass spectrometry techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef]
- Carrara, M.; Kelly, M.T.; Roso, F.; Larroque, M.; Margout, D. Potential of olive oil mill wastewater as a source of polyphenols for the treatment of skin disorders: A review. J. Agric. Food Chem. 2021, 69, 7268–7284. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2014, 3, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, G.; Novelli, E.; Taticchi, A.; Servili, M. Application of recovered bioactive compounds in food products. In Olive Mill Waste Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 231–253. [Google Scholar]
- Ianni, F.; Gagliardi, A.; Taticchi, A.; Servili, M.; Pinna, N.; Schoubben, A.; Sardella, R.; Bruscoli, S. Exploiting food-grade mesoporous silica to preserve the antioxidant properties of fresh olive mill wastewaters phenolic extracts. Antioxidants 2021, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Martínez-Carballo, E.; Cambeiro-Pérez, N.; Rial-Otero, R.; Figueiredo-González, M.; Cancho-Grande, B. Applicability of an in-vitro digestion model to assess the bioaccessibility of phenolic compounds from olive-related products. Molecules 2021, 26, 6667. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Taticchi, A.; Esposto, S.; Servili, M.; Ranucci, D.; Branciari, R.; Miraglia, D. The influence of phenolic extract from olive vegetation water and storage temperature on the survival of Salmonella Enteritidis inoculated on mayonnaise. LWT 2020, 129, 109648. [Google Scholar] [CrossRef]
- Barbieri, S.; Mercatante, D.; Balzan, S.; Esposto, S.; Cardenia, V.; Servili, M.; Novelli, E.; Taticchi, A.; Rodriguez-Estrada, M.T. Improved oxidative stability and sensory quality of beef hamburgers enriched with a phenolic extract from olive vegetation water. Antioxidants 2021, 10, 1969. [Google Scholar] [CrossRef]
- Gayoso, L.; Roxo, M.; Cavero, R.Y.; Calvo, M.I.; Ansorena, D.; Astiasaran, I.; Wink, M. Bioaccessibility and biological activity of Melissa officinalis, Lavandula latifolia and Origanum vulgare extracts: Influence of an in vitro gastrointestinal digestion. J. Funct. Foods 2018, 44, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic compounds of blackthorn (Prunus spinosa L.) and influence of in vitro digestion on their antioxidant capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Olmo-García, L.; Bajoub, A.; Benlamaalam, S.; Hurtado-Fernández, E.; Bagur-González, M.G.; Chigr, M.; Mbarky, M.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Establishing the phenolic composition of Olea europaea L. leaves from cultivars grown in Morocco as a crucial step towards their subsequent exploitation. Molecules 2018, 23, 2524. [Google Scholar] [CrossRef] [Green Version]
- Ladhari, A.; Zarrelli, A.; Ghannem, M.; Ben Mimoun, M. Olive wastes as a high-potential by-product: Variability of their phenolic profiles, antioxidant and phytotoxic properties. Waste Biomass Valorization 2021, 12, 3657–3669. [Google Scholar] [CrossRef]
- Günal-Köroğlu, D.; Turan, S.Y.; Kıralan, M.; Ramadan, M.F. Enhancement of sunflower oil stability during deep-frying using extracts from olive oil by-products and soy lecithin. Int. Food Res. J. 2019, 26, 1269–1277. [Google Scholar]
- Taticchi, A.; Esposto, S.; Urbani, S.; Veneziani, G.; Selvaggini, R.; Sordini, B.; Servili, M. Effect of an olive phenolic extract added to the oily phase of a tomato sauce, on the preservation of phenols and carotenoids during domestic cooking. LWT Food Sci. Technol. 2017, 84, 572–578. [Google Scholar] [CrossRef]
- Miraglia, D.; Castrica, M.; Menchetti, L.; Esposto, S.; Branciari, R.; Ranucci, D.; Urbani, S.; Sordini, B.; Veneziani, G.; Servili, M. Effect of an olive vegetation water phenolic extract on the physico-chemical, microbiological and sensory traits of shrimp (Parapenaeus longirostris) during the shelf-life. Foods 2020, 9, 1647. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Geraldi, M.V.; do Nascimento, R.P.; Moya, A.M.T.M.; Vezza, T.; Diez-Echave, P.; Gálvez, J.J.; Cazarin, C.B.B.; Maróstica Júnior, M.R. Polyphenols from food by-products: An alternative or complementary therapy to IBD conventional treatments. Food Res. Int. 2021, 140, 110018. [Google Scholar] [CrossRef]
- Dauber, C.; Carreras, T.; Fernández, A.F.; Irigaray, B.; Albores, S.; Gámbaro, A.; Ibanez, E.; Vieitez, I. Response surface methodology for the optimization of biophenols recovery from “alperujo” using supercritical fluid extraction. Comparison between Arbequina and Coratina cultivars. J. Supercrit. Fluids 2022, 180, 105460. [Google Scholar] [CrossRef]
- Burgos-Edwards, A.; Jiménez-Aspee, F.; Thomas-Valdés, S.; Schmeda-Hirschmann, G.; Theoduloz, C. Qualitative and quantitative changes in polyphenol composition and bioactivity of Ribes magellanicum and R. punctatum after in vitro gastrointestinal digestion. Food Chem. 2017, 237, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Pavan, V.; Sancho, A.R.S.; Pastore, G.M. The effect of in vitro digestion on the antioxidant activity of fruit extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). LWT Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar] [CrossRef] [Green Version]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Casciano, F.; Gianotti, A. Intestinal fermentation in vitro models to study food-induced gut microbiota shift: An updated review. FEMS Microbiol. Lett. 2020, 367, fnaa097. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]



| Enzymes | Concentration | Solvent | Added Quantity | |
|---|---|---|---|---|
| Oral phase | α-amylase | 1.3 mg/mL | CaCl2 1 mM | 125 μL |
| Gastric phase | Pepsin | 160 mg/mL | HCl 0.1 M | 165 μL |
| Intestinal phase | Pancreatin solution: bile extract (1:1, v/v) | Pancreatin solution (4 mg/mL); bile extract (25 mg/mL) | NaHCO3 0.1 M | 1250 μL |
| 3,4-DHPEA | p-HPEA | 3,4-DHPEA-EDA | p-HPEA-EDA | VB | Total Phenols | ||
|---|---|---|---|---|---|---|---|
| A20 | ND | 12.56 ± 0.38 b,B | 3.81 ± 0.21 b,A | 72.40 ± 0.48 a,A | 9.50 ± 0.19 a,A | 2.52 ± 0.06 a,A | 100.81 ± 7.35 a,A |
| OD | 17.89 ± 0.83 a,B | 7.32 ± 0.40 a,A | 46.35 ± 0.47 b,A | 7.25 ± 0.20 b,A | 1.39 ± 0.22 c,A | 80.22 ± 11.10 b,A | |
| GD | 5.69 ± 0.26 c,B | 2.47 ± 0.39 c,A | 49.20 ± 0.57 b,A | 6.97 ± 0.24 c,A | 1.95 ± 0.05 b,A | 66.31 ± 8.18 c,A | |
| ID | 5.50 ± 0.13 c,C | n.d. d,C | 3.89 ± 0.07 c,B | n.d. d,B | n.d. d,C | 9.39 ± 1.59 d,B | |
| A21 | ND | 14.76 ± 1.78 b,A | 2.16 ± 0.07 a,B | 53.15 ± 5.64 a,B | 3.58 ± 0.11 a,B | 0.63 ± 0.03 a,C | 74.29 ± 7.76 a,B |
| OD | 31.10 ± 1.15 a,A | 2.41 ± 0.12 a,B | 33.60 ± 1.36 b,B | 2.30 ± 0.13 b,B | 0.50 ± 0.01 b,C | 69.64 ± 7.68 b,B | |
| GD | 8.87 ± 0.41 d,A | 0.88 ± 0.02 b,B | 12.32 ± 1.11 c,B | 1.04 ± 0.02 c,B | 0.37 ± 0.01 c,C | 23.50 ± 1.48 c,B | |
| ID | 9.15 ± 0.16 c,A | 0.96 ± 0.02 b,A | 6.22 ± 0.74 d,A | 0.73 ± 0.01 d,A | 0.05 ± 0.00 d,B | 17.14 ± 1.92 d,A | |
| SP | ND | 6.64 ± 0.67 c,C | 0.20 ± 0.01 c,C | 10.77 ± 0.06 a,C | n.d. C | 1.10 ± 28.99 a,B | 18.37 ± 6.84 a,C |
| OD | 7.18 ± 0.50 b,C | 0.21 ± 0.06 c,C | n.d. c,C | n.d. C | 0.91 ± 15.69 b,B | 8.31 ± 0.51 d,C | |
| GD | 5.18 ± 0.01 d,B | 0.30 ± 0.01 b,C | 3.93 ± 0.01 b,C | n.d. C | 0.82 ± 12.19 c,B | 10.25 ± 0.97 b,C | |
| ID | 8.03 ± 0.07 a,B | 0.40 ± 0.07 a,B | n.d. c,C | n.d. B | 0.78 ± 14.88 d,A | 9.22 ± 0.70 c,B |
| 15 | 30 | 45 | 60 | 75 | 90 | ||
|---|---|---|---|---|---|---|---|
| Min | |||||||
| A20 | ND | 2.64 ± 0.21 c,C | 2.14 ± 0.16 d,C | 2.41 ± 0.23 c,C | 2.25 ± 0.23 d,C | 2.18 ± 0.23 c,C | 2.11 ± 0.22 c,C |
| OD | 17.75 ± 1.64 b,C | 17.67 ± 2.14 c,B | 19.14 ± 2.95 b,B | 17.09 ± 2.95 c,B | 15.41 ± 1.55 b,B | 15.17 ± 1.03 b,B | |
| GD | 22.47 ± 0.87 a,C | 21.47 ± 0.80 b,B | 21.27 ± 1.20 b,B | 21.33 ± 1.20 b,B | 21.23 ± 1.41 a,B | 21.40 ± 1.43 a,B | |
| ID | 0 d,B | 36.74 ± 0.00 a,B | 31.52 ± 1.70 a,B | 27.81 ± 1.70 a,B | 0 d,B | 0 d,B | |
| A21 | ND | 7.36 ± 0.54 b,B | 6.91 ± 0.40 c,B | 6.56 ± 0.29 c,B | 6.21 ± 0.26 c,B | 5.93 ± 0.28 c,B | 5.64 ± 0.29 c,B |
| OD | 25.35 ± 2.14 a,B | 15.53 ± 1.73 b,B | 15.47 ± 2.09 b,C | 14.17 ± 1.29 bC, | 14.43 ± 1.65 b,C | 13.70 ± 0.96 b,C | |
| GD | 22.47 ± 1.57 a,C | 21.47 ± 0.85 a,B | 21.27 ± 1.27 a,B | 21.33 ± 1.25 a,B | 21.23 ± 1.26 a,B | 21.40 ± 1.38 a,B | |
| ID | 0 c,B | 0 d,C | 0 d,C | 0 d,C | 0 d,B | 0 d,B | |
| SP | ND | 169.08 ± 20.31 a,A | 156.06 ± 15.91 a,A | 151.85 ± 13.08 a,A | 147.62 ± 10.71 a,A | 159.11 ± 10.29 a,A | 159.59 ± 12.05 a,A |
| OD | 102.24 ± 14.99 c,A | 96.90 ± 15.85 c,A | 95.73 ± 16.74 c,A | 94.48 ± 16.89 c,A | 96.66 ± 16.95 c,A | 99.39 ± 15.70 c,A | |
| GD | 143.29 ± 10.12 b,A | 131.03 ± 8.24 b,A | 128.86 ± 7.87 b,A | 131.68 ± 7.35 b,A | 133.94 ± 7.71 b,A | 134.77 ± 7.06 b,A | |
| ID | 53.36 ± 1.69 d,A | 49.48 ± 1.70 d,A | 49.87 ± 2.01 d,A | 52.52 ± 2.11 d,A | 53.99 ± 2.78 d,A | 75.34 ± 12.14 d,A | |
| TP | 3,4-DHPEA | p-HPEA | 3,4-DHPEA-EDA | p-HPEA-EDA | VB | Total Phenols | ABTS•+ | AAPH | IC50 15 Min | IC50 30 Min | IC50 45 Min | IC50 60 Min | IC50 75 Min | IC50 90 Min | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | - | ||||||||||||||
| 3,4-DHPEA | 0.503 | - | |||||||||||||
| p-HPEA | 0.777 * | 0.521 | - | ||||||||||||
| 3,4-DHPEA-EDA | 0.843 * | 0.437 * | 0.745 * | - | |||||||||||
| p-HPEA-EDA | 0.809 * | 0.294 | 0.827 * | 0.935 * | - | ||||||||||
| VB | 0.433 | −0.019 | 0.525 | 0.703 * | 0.808 * | - | |||||||||
| Total Phenols | 0.863 * | 0.595 * | 0.813 * | 0.980 * | 0.915 * | 0.645 * | - | ||||||||
| ABTS•+ | 0.910 * | 0.462 | 0.645 * | 0.797 * | 0.663 * | 0.243 | 0.794 * | - | |||||||
| AAPH | 0.666 * | 0.370 | 0.380 | 0.645 * | 0.413 | 0.051 | 0.618 * | 0.910 * | - | ||||||
| IC50 15 min | −0.613 * | −0.344 | −0.427 | −0.476 | −0.481 | 0.040 | −0.497 | −0.624 * | −0.485 | - | |||||
| IC50 30 min | −0.685 * | −0.436 | −0.492 | −0.546 | −0.539 | −0.029 | −0.580 * | −0.680 * | −0.528 | 0.979 * | - | ||||
| IC50 45 min | −0.678 * | −0.429 | −0.479 | −0.540 | −0.531 | −0.016 | −0.572 | −0.676 * | −0.528 | 0.984 * | 1.000* | - | |||
| IC50 60 min | −0.682 * | −0.435 | −0.485 | −0.544 | −0.533 | −0.013 | −0.576 * | −0.679 * | −0.530 | 0.986 * | 0.998 * | 0.999 * | - | ||
| IC50 75 min | −0.627 * | −0.387 | −0.438 | −0.488 | −0.484 | 0.046 | −0.516 | −0.633 * | −0.492 | 0.999 * | 0.981 * | 0.986 * | 0.989 * | - | |
| IC50 90 min | −0.654 * | −0.400 | −0.457 | −0.517 | −0.505 | 0.039 | −0.544 | −0.660 * | −0.514 | 0.990 * | 0.972 * | 0.977 * | 0.982 * | 0.994 * | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercatante, D.; Ansorena, D.; Taticchi, A.; Astiasarán, I.; Servili, M.; Rodriguez-Estrada, M.T. Effects of In Vitro Digestion on the Antioxidant Activity of Three Phenolic Extracts from Olive Mill Wastewaters. Antioxidants 2023, 12, 22. https://doi.org/10.3390/antiox12010022
Mercatante D, Ansorena D, Taticchi A, Astiasarán I, Servili M, Rodriguez-Estrada MT. Effects of In Vitro Digestion on the Antioxidant Activity of Three Phenolic Extracts from Olive Mill Wastewaters. Antioxidants. 2023; 12(1):22. https://doi.org/10.3390/antiox12010022
Chicago/Turabian StyleMercatante, Dario, Diana Ansorena, Agnese Taticchi, Iciar Astiasarán, Maurizio Servili, and Maria Teresa Rodriguez-Estrada. 2023. "Effects of In Vitro Digestion on the Antioxidant Activity of Three Phenolic Extracts from Olive Mill Wastewaters" Antioxidants 12, no. 1: 22. https://doi.org/10.3390/antiox12010022